AHL Newsletter, March 2015
For a pdf copy of this Newsletter please click here
Welcome Dr. Véronique LePage, aquatic animal pathologist
Dr. Véronique (Nikki) LePage has joined us as our AHL contract fish pathologist, working mostly from her home office. Nikki is a Guelph graduate (BSc 2006, DVM 2011, MSc 2012) and is familiar to many in the aquaculture industry.
The fish pathology lab has been transferred from Dr. John Lumsden to the AHL, and our primary focus will be on Ontario farmed fish - trout, tilapia, char - but Nikki has broader interests, and we expect this scope to grow. Fish cases are processed through accessioning in our LIMS through to the AHL Molecular Biology lab for gross pathology, bacteriology, and virology. Nikki will add histopathology and case integration.
As noted in the December 2014 AHL Newsletter, we have validated identification tests available for aquatic animal bacteria and viral hemorrhagic septicemia virus.
Welcome Nikki!

Beware formalin fumes!
Kristiina Ruotsalo
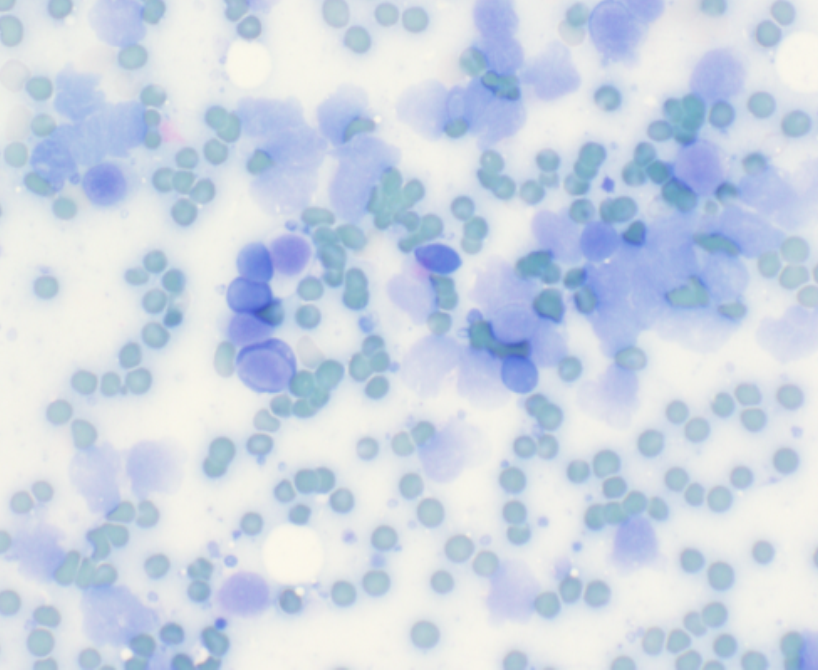
Unstained cytology and hematology slides should never be transported with, or stored near, tissue samples contained in formalin. Formalin fumes can penetrate almost any packaging, even biopsy samples enclosed in plastic jars with screw-top lids that are sealed in plastic bags. Exposure of unstained slide preparations to formalin fumes results in altered staining characteristics and poor cellular preservation due to the partial fixation of cellular material by the formalin fumes. Subsequent staining of the slides reveals the characteristic blue-green tinged erythrocytes and lysed nucleated cells, making slide interpretation impossible.
|
|
|
|
Air-dried, well-stained blood smear |
Cytology smear exposed to formalin fumes before staining. |
Selected AHL outreach presentations, 2014
Barham M, Vince A, et al. 6 OAHN podcasts: small ruminant, equine, swine, rabies. Fall 2014. http://barhamm.podbean.com/.
Brash M. Diagnostic Pathology Submissions to the AHL. Parrot Conference and Expo Canada. Cambridge, ON. April 12, 2014.
Brash M. 2014 Canadian Food Inspection Agency Veterinary Professional Update Course – Poultry Health Update. Ontario Veterinary College, Guelph, ON. May 27, 2014.
Brash M, Martin E, Stalker M, Hoyland S, Coventry J, Sandrock C, Ojkic D. Clinical & Pathological Features of IBV 4/91 Infection in Ontario’s Commercial Poultry. WAPV Scientific Seminar. Banff, AB. Oct 7, 2014.
Brooks A. Hepatic alveolar echinococcosis in a dog in Ontario. Ann CAHLN-RCTLSA Meeting, Ottawa. July 2014.
Brooks A. Cache valley virus causing lamb malformations in Ontario. Annual CAVP-ACPV Meeting, Ottawa, ON, June 1, 2014.
Brooks A. Lyme borreliosis in a Labrador retriever. Annual CAVP-ACPV Meeting, Ottawa, ON, June 1, 2014.
Brooks A, Ruotsalo K. Bovine anaplasmosis in eastern Ontario. Annual CAVP-ACPV Meeting, Ottawa ON, June 1, 2014.
Fairles J, Ojkic D, Hazlett M, Maxie G. PEDV – A case study of one lab's response to an emerging disease. CAHLN-RCTLSA annual mtg, OLF, Nepean ON. June 3, 2014; and CAVP-ACPV annual meeting, Ottawa ON. June1, 2014.
Fournier D, Venne D, Lejeune M, Brash M. Oh my aching head. A broiler chicken’s tale of woe. WAPV Scientific Seminar. Banff, AB. Oct 7, 2014.
Hazlett MJ. Food Animal Diagnostic Pathology – Diseases of Swine. 4th year OVC students. Dec 11, 2014.
Martin E, Brash M, Reid A. White striping in breast muscles a.k.a. 'wooden breast'. OAPP meeting, Guelph ON. Nov27, 2014.
Maxie G, Alves D, Pasma T, McNab B. DSP – Disease Surveillance Program – the Ontario plan. CAHLN-RCTLSA annual mtg, OLF, Nepean, ON. June 2, 2014.
Maxie G, Cai H, Ojkic D, Slavic D, DeLay J, Barham M. Veterinary laboratory knowledge translation and transfer. AAVLD annual meeting. Kansas City, MO. Oct 18, 2014.
Maxie G, Fairles J, Ojkic D. PEDV and PDCoV: Canadian perspective. Laboratory Directors Committee, AAVLD annual meeting. Kansas City, MO. Oct 18, 2014.
McEwen BJ. Veterinary Forensic Pathology: I. State of veterinary pathology & Lessons learned from a precedent setting case. II Expectations of investigators & what to expect being an expert witness. III Asphyxia (non-drowning) & Méli-Mélo (aging of lesions, postmortem interval. Programme Annuel de Formation Continue, Les Laboratoires de Pathologie Vétérinaires, MAPAQ, St Hyacinthe, Quebec. October 26-27, 2014.
McEwen BJ. Veterinary Forensic Pathology. CAVP-ACPV Annual Meeting, Ottawa, ON. June 1, 2014.
McEwen BJ, DeLay J. Jugular Vein Lesions in Racehorses. International Veterinary Forensic Sciences Association Annual Meeting, Orlando, Florida. May 25, 2014.
Ojkic D, Hazlett M, Fairles J, Marom A, Slavic D, Maxie G, Alexandersen S, Pasick J, Alsop J, Burlatschenko S. Porcine Epidemic Diarrhea Update. Mike Wilson Research Day. Arboretum, Guelph, ON. June 4, 2014.
Shapiro J. Malignant catarrhal fever in swine in eastern Ontario. CAVP-ACPV Annual Meeting, Ottawa, ON. June 1, 2014.
Sunohara-Neilson J, Nagy E, Brash M, Tapscott B, Turner PV. Development of diagnostic tests for Leporid herpesvirus 4 infection of Ontario Rabbits. 2014 Ontario Food Safety Research Forum, Guelph, ON. May 8, 2014.
Varga C. Brash M, Barham M. Ontario Poultry Health Update. 2014 Poultry Producer Updates, OMAFRA & PIC. Brodhagen, ON. Dec 10, 2014.
Zechel J, Cai, H. Honey bee testing at the Animal Health Laboratory. Ontario Beekeeper's Association Annual General Meeting, Markham, ON. Oral presentation. Nov 20-21, 2014.
Selected zoonotic pathogens and diseases from Ontario identified at the AHL, 2014
Beverly McEwen, Durda Slavic, Davor Ojkic, Josepha DeLay, Hugh Cai, Margaret Stalker, Murray Hazlett, Andrew Brooks, Kristiina Ruotsalo, Jan Shapiro
Many new, emerging, and re-emerging diseases of people are caused by pathogens originating from animals, or are shared between people and animals. The AHL plays an important role in public health by identifying zoonotic pathogens in about 1,000 cases annually (Tables 1 and 2). These are numerator data reliant upon submission biases to the diagnostic laboratory and cannot be regarded as population prevalence estimates. Monitoring programs are not included.
Table 1. Cases with selected zoonotic pathogens isolated and/or identified at the AHL, 2014
|
Agent |
Bovine |
Swine |
Equine |
Ovine |
Caprine |
Chicken |
Turkey |
Canine |
Feline |
Other |
2014 |
2013 |
2012 |
2011 |
2010 |
2009 |
|
Ascarids (T. canis, T. cati, T. leonina, Baylisascaris sp.) |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
23 |
14 |
3 |
40 |
36 |
35 |
ND |
ND |
ND |
|
Blastomyces dermatitidis |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
19 |
0 |
3 |
22 |
17 |
10 |
10 |
5 |
10 |
|
Bordetella bronchiseptica |
0 |
10 |
1 |
0 |
0 |
0 |
0 |
5 |
5 |
7 |
28 |
24 |
33 |
43 |
54 |
60 |
|
Borrelia burgdorferi (Lyme disease), serology |
0 |
0 |
7 |
0 |
0 |
0 |
0 |
5 |
0 |
0 |
12 |
11 |
3 |
1 |
|
|
|
Brucella sp. (non-abortus) |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
0 |
0 |
1 |
0 |
0 |
0 |
0 |
0 |
|
Campylobacter coli/ jejuni/ fetus subsp. fetus |
3 |
0 |
0 |
9 |
0 |
0 |
0 |
3 |
1 |
1 |
17 |
6 |
17 |
12 |
24 |
14 |
|
Chlamydophila sp. (C. abortus except 1 C. psittaci in a bird) |
0 |
0 |
0 |
5 |
9 |
0 |
0 |
0 |
0 |
1 |
15 |
25 |
33 |
39 |
58 |
29 |
|
Clostridium difficile |
1 |
7 |
2 |
0 |
0 |
0 |
0 |
1 |
0 |
0 |
11 |
11 |
19 |
40 |
31 |
24 |
|
Coxiella burnetii (Q fever) |
6 |
0 |
0 |
25 |
23 |
0 |
0 |
0 |
0 |
1 |
55 |
28 |
36 |
99 |
115 |
9 |
|
Cryptococcus sp. |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
2 |
1 |
3 |
2 |
1 |
|
|
|
|
Cryptosporidium sp. |
155 |
3 |
0 |
5 |
13 |
0 |
0 |
0 |
0 |
10 |
186 |
206 |
141 |
147 |
157 |
128 |
|
Eastern equine encephalitis virus |
0 |
0 |
24 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
25 |
1 |
0 |
5 |
12 |
11 |
|
Giardia sp. |
8 |
0 |
0 |
2 |
0 |
0 |
0 |
30 |
10 |
0 |
50 |
48 |
26 |
31 |
60 |
55 |
|
Listeria monocytogenes |
12 |
0 |
0 |
4 |
6 |
0 |
0 |
0 |
0 |
1 |
23 |
15 |
18 |
18 |
19 |
18 |
|
Methicillin-resistant Staphylococcus aureus (MRSA) |
0 |
2 |
10 |
0 |
0 |
2 |
0 |
1 |
2 |
0 |
17 |
8 |
24 |
49 |
74 |
36 |
|
Methicillin-resistant Staphylococcus pseudintermedius (MRSP) |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
45 |
0 |
0 |
45 |
141 |
114 |
192 |
ND |
ND |
|
Rabies |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
3 |
8 |
|
Salmonella enterica |
70 |
68 |
3 |
3 |
1 |
34 |
20 |
2 |
0 |
20 |
221 |
308 |
281 |
256 |
256 |
281 |
|
Streptococcus suis |
23 |
76 |
0 |
3 |
2 |
0 |
0 |
0 |
1 |
0 |
105 |
126 |
144 |
106 |
110 |
120 |
|
Streptococcus equisimilis |
0 |
0 |
3 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
4 |
34 |
45 |
59 |
48 |
43 |
|
Streptococcus zooepidemicus |
2 |
0 |
86 |
0 |
0 |
0 |
0 |
0 |
4 |
1 |
93 |
112 |
4 |
149 |
152 |
117 |
|
Toxoplasma sp. |
0 |
0 |
0 |
13 |
1 |
0 |
0 |
0 |
4 |
0 |
18 |
11 |
8 |
24 |
22 |
19 |
|
Verotoxigenic E.coli (VTEC) |
6 |
0 |
0 |
0 |
1 |
0 |
0 |
0 |
0 |
0 |
7 |
18 |
|
|
|
|
|
West Nile virus |
0 |
0 |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
5 |
6 |
44 |
36 |
34 |
7 |
6 |
|
Yersinia enterocolitica |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
0 |
4 |
6 |
4 |
2 |
1 |
2 |
0 |
|
Total |
287 |
166 |
137 |
69 |
56 |
36 |
20 |
136 |
43 |
60 |
1010 |
1236 |
1043 |
1315 |
1209 |
988 |
Table 2. Leptospira spp. seropositive and IHC positive cases identified at the AHL, 2014
|
Leptospira spp. serovar* |
Bovine |
Swine |
Equine |
Canine |
Other & not specified |
|
L. autumnalis |
17 |
1 |
11 |
33 |
1 |
|
L. bratislava |
23 |
3 |
9 |
10 |
1 |
|
L .canicola |
27 |
0 |
4 |
14 |
0 |
|
L. grippotyphosa |
7 |
1 |
3 |
25 |
0 |
|
L. hardjo |
24 |
0 |
1 |
3 |
0 |
|
L. icterohaemorrhagiae |
25 |
1 |
8 |
20 |
1 |
|
L. pomona |
33 |
1 |
6 |
14 |
1 |
|
IHC or urine PCR positive |
0 |
0 |
0 |
1 |
0 |
RUMINANTS
Verotoxigenic E.coli associated with diarrhea and mortality in young calves
Andrew Brooks, Durda Slavic, Margaret Stalker, Murray Hazlett, Maria Spinato, Beverly McEwen
VTEC are a heterogeneous group of E.coli that express one or more Shiga toxins (stx). VTEC have important public health significance because some strains cause hemorrhagic colitis and hemolytic uremia syndrome in people. VTEC are carried subclinically in the intestine of healthy cattle, which are an important reservoir for human infection, and sporadically cause diarrhea and dysentery in young calves.
Verotoxigenic E.coli (VTEC) was determined to be the cause of diarrhea or death in 9 calves submitted to the AHL for postmortem or histological examination from January 2013 to December 2014. The major clinical signs were diarrhea and sudden death in calves 4-30 days of age (Table 1). The most common gross lesion was enterocolitis that was often mild; in some cases fibrinonecrotic or fibrinohemorrhagic intestinal exudates were present. The common histologic lesion was attachment of bacilli to the apical border of small intestinal or colonic enterocytes (Fig. 1). E. coli isolates from the intestine (1 isolate was from feces) were genotyped at the AHL with a PCR assay that detects the intimin (eaeA), hemolysin (hlyA), and Shiga toxin 1 and 2 (stx1/2) virulence genes. The majority of isolates from these cases were positive for eaeA, hlyA, and stx1. Co-infections with other pathogens associated with calf diarrhea were common.

Figure 1. VTEC attached to surface enterocytes of the small intestine (from case 4).
Table 1. Case summaries of diarrhea and mortality in calves associated with VTEC infection
|
Case |
Breed |
Age (days) |
Major clinical problems |
Major postmortem lesions |
E. coli virulence factor genotype |
Co-infections |
|||
|
eaeA hylA stx1 stx2 |
|||||||||
|
1 |
Holstein |
4 |
Bloody feces Found dead |
Fibrinonecrotic enteritis |
+ |
+ |
+ |
- |
Cryptosporidium |
|
2* |
not given |
10 |
Diarrhea |
Erosive enterocolitis |
+ |
+ |
+ |
- |
Cryptosporidium |
|
3 |
Holstein |
10 |
Diarrhea, pneumonia |
Enterocolitis, mycotic rumenitis |
+/- 1 |
+/- |
+/- |
- |
Enterotoxigenic E. coli Cryptosporidium Coronavirus |
|
4 |
Mixed-beef |
9 |
Diarrhea, sudden death |
Enterocolitis Septicemia |
+ |
+ |
+ |
- |
Rotavirus |
|
5 |
Charolais |
14 |
Diarrhea |
Enterocolitis Mycotic rumenitis/reticulitis |
+ |
+ |
+ |
- |
Rotavirus |
|
6 |
Holstein |
30 |
Found dead |
Enteritis, rumenitis, meningitis, pyelonephritis |
+ |
+ |
+ |
- |
- |
|
7 |
Jersey |
15 |
Diarrhea |
Enterocolitis, abomasitis, rumenitis |
+ |
+ |
+ |
- |
Cryptosporidium Rotavirus |
|
8 |
Holstein |
3 |
Found dead |
Fibrino-hemorrhagic enteritis, abomasitis |
- |
+ |
+ |
+ |
- |
|
9 |
Holstein |
10 |
Acute death |
Enterocolitis, abomasitis |
+/- |
+ |
+ |
- |
Coronavirus Salmonella Muenster Cryptosporidium |
* Field postmortem; 1. +/- denotes suspicious PCR result.
An outbreak of Campylobacter jejuni abortions in a small dairy herd
Maria Spinato, Andrew Vince, Durda Slavic, Geert Jongert
The producer of a closed, well-managed herd of 85 Holstein cattle reported 9 abortions during a 2-week period. Cattle are vaccinated with a modified live, 5-way vaccine (IBRV, BVDV types 1 and 2, BRSV, PI3V) at freshening. Most of the aborted fetuses were between 200-260 days gestation. Three fetuses and placentas were submitted to the AHL for postmortem examination and ancillary testing. Placentas contained variably-sized, relatively uniform tan cotyledons; slight marginal cupping and congestion were noted in several cotyledons in the placenta of fetus 3. All 3 fetuses were moderately autolysed. The only remarkable observation in fetal tissues was the finding of an edematous sheet of fibrin overlying the lung of fetus 3. Fibrinous pleuritis is most often observed in abortions caused by bacterial infections; therefore, Campylobacter culture and Leptospira MAT were performed, in addition to routine aerobic bacterial culture. Campylobacter jejuni subsp. jejuni was isolated in one or more samples of lung, abomasal fluid and placenta cultured from all 3 fetuses. Leptospira MATs and PCR tests for BVDV, BoHV-1 (IBRV), and Coxiella burnetii were negative. Neospora caninum ELISAs performed on maternal sera of fetuses 1 and 2 were also negative. Histologic lesions included multifocal neutrophilic necrotizing placentitis, colonization of chorionic stroma and occasional trophoblastic epithelial cells by abundant Gram negative bacteria, and hypercellular alveolar septa in lung sections due to circulating neutrophils. The allantois of fetus 1 also had a few arterioles characterized by hyaline walls and fibrin thrombi.
Upon further investigation, it was discovered that a cow had aborted on this farm approximately 2.5-3 weeks prior to the outbreak. This cow was housed in a hospital pen that is scraped out twice weekly using the same tractor and bucket used for making the total mixed ration (TMR). Although the bucket was rinsed with water after scraping the hospital pen, it was not thoroughly cleaned or disinfected. It is suspected that this abortion was the index case (not submitted for testing), and that the placenta contaminated the TMR which was the source of infection for the other aborting cows via ingestion. Subsequent to this outbreak, a premature live calf and a first trimester abortion at 60 days gestation were reported on this farm; however, no additional testing was performed and their relation to the outbreak could not be confirmed.
Campylobacter jejuni is a normal commensal organism found in the gastrointestinal tract of ruminants and other food-producing animals. Although sporadic abortions have been reported in cattle, abortion outbreaks due to C. jejuni are extremely rare. Conversely, outbreaks of C. jejuni abortion in small ruminants are a more common occurrence. The emergence of tetracycline-resistant strains in Canada and the USA is of significant concern to the small ruminant industries, as tetracycline is used both therapeutically and prophylactically for infectious causes of abortion (1). Minimum inhibitory concentration (MIC) analysis of the C. jejuni isolate in this case confirmed that it was susceptible to tetracycline.
This case highlights the importance of expanding on-farm biosecurity protocols to include appropriate disposal and disinfection procedures for aborted fetuses and placentas. Many abortifacient bacterial species in ruminants also pose a significant zoonotic risk for producers, their families and veterinarians. Campylobacter jejuni is recognized world-wide as a significant cause of gastroenteritis in humans, and contamination of food and water supplies by bovine feces is a known risk factor.
An alternative method for brain removal
Josepha DeLay, Andrew Brooks
An alternative approach and, for large animals especially, often easier method to expose brain is by using a lateral approach, cutting through a coronal plane.


Figure 1. Landmarks for brain removal by lateral approach. Figure 6. Brain removal in rostral and caudal sections. These may be sagittally sectioned, with half of each section fixed in
formalin for histopathology, and the remaining halves stored fresh or frozen for microbiologic tests.
Field and clinic postmortems. I: Simplified protocol and image list
Josepha DeLay
Digital images captured during postmortem (PM) examinations provide a permanent record of lesions. The images are a very useful communication tool when consulting with pathologists and other specialists. PM images provide valuable supplemental information for pathologists evaluating tissue samples submitted to a diagnostic laboratory for histologic examination. Images may be emailed to AHL pathologists at ahlpath@uoguelph.ca
The image list below provides both a step-wise guide to the postmortem procedure and a suggested set of images that are applicable to all species of companion and food-producing animals. Establishing and following a standard routine for PM procedures is important. This allows the practitioner to spend more time identifying and interpreting lesions, rather than concentrating on the logistics of the exam. Developing a PM routine is similar to having a routine protocol for physical examination in a live patient.
Remove ear tag or create ID label, and include with all photos.
Image 1. External views: full body, head, thorax / abdomen, perineum
- for unexpected deaths, take image in situ, in location and position where body was discovered.
- include views that depict body condition, hydration (eyes), evidence of predation or trauma, etc.
Open abdominal and thoracic cavities.
Image 2. Opened thorax (with organs in situ).
Image 3. Heart in situ, with pericardial sac opened (check for fluid, exudate, etc.).
Remove pluck.
Image 4. Pluck, with focus on lungs (dorsoventral view, with right and left lung visible).
Image 5. Cross-section of right and left lung.
Image 6. Cross-section of heart through both ventricles.
Image 7. Larynx (including thyroid glands) and trachea: opened and mucosal surface exposed.
Image 8. Opened abdomen (with organs in situ).
In ruminants, remove omentum. In all species, fan out intestines and locate cecum and ileum.
Image 9. Opened abdomen with intestines fanned out.
Image 10. Open cecum, ileum, and jejunum to expose mucosal surface.
Image 11. Open colon to expose mucosal surface.
Image 12. Open duodenum to expose mucosal surface.
Image 13. Liver – capsular surface. For ruminants, include opened caudal vena cava.
Image 14. Liver – cross section.
Image 15. Abomasum / stomach – serosal surface.
Image 16. Abomasum / stomach – mucosal surface.
Image 17. Ruminants: rumen – serosal surface.
Image 18. Ruminants: rumen – mucosal surface and content.
Image 19. Solid organs:
- kidneys: sagittal sections, with cut surfaces exposed
- spleen: cross section
- adrenal glands
Unexpected death / neurologic cases: Remove brain. Also remove spinal cord if required, based on clinical signs.
Image 20. Brain. The next installment in this series will focus on ancillary test selection and sample collection during PM exams.
Theileriosis in a dairy cow from eastern Ontario
Kris Ruotsalo, Jan Shapiro
A 3-year-old Holstein cow from eastern Ontario had a 2-month clinical history of pyrexia, diarrhea, lameness, and weight loss. Significant hematological changes included marked, mildly responsive anemia; hematocrit 0.15 L/L (reference interval 0.21-0.30 L/L), hemoglobin 48 g/L (reference interval 84-120 g/L), and mild lymphopenia. Significant biochemistry changes included hypoproteinemia (total protein 50 g/L) and hyperbilirubinemia (total bilirubin 20 µmol/L).
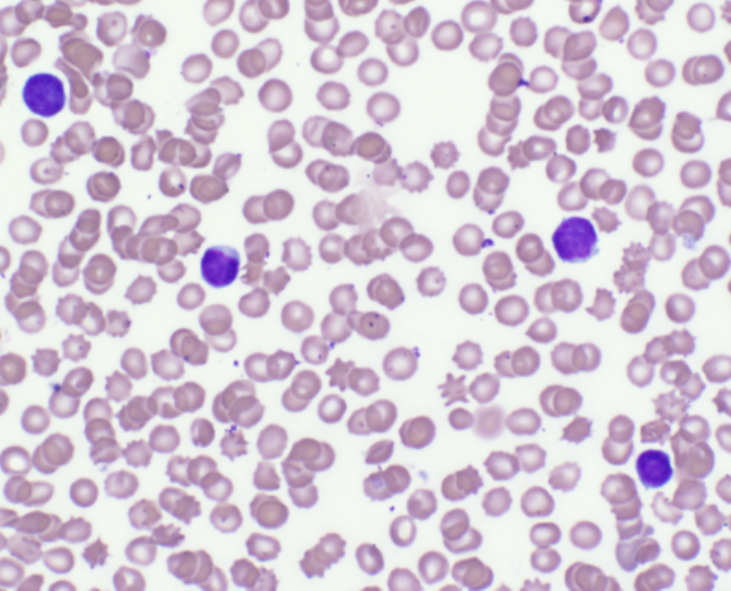
Peripheral blood smear examination revealed numerous intraerythrocytic organisms (Fig. 1). These organisms were pleomorphic, exhibiting round, rod, comma, and signet ring forms. A tentative diagnosis of Theileria spp. infection was made. No commercially available test to confirm theileriosis is available, and the species of Theileria cannot be morphologically distinguished on the basis of a blood smear. Confirmatory testing by 18S RNA amplification and gene sequence analysis was performed at the Animal Health Diagnostic Laboratory, Cornell University, Ithaca, NY. Analysis revealed that the Theileria organisms within this blood sample were part of the Theileria buffeli complex.
The affected cow continued to deteriorate clinically, and euthanasia was elected. Repeated CBC analysis just prior to euthanasia revealed the ongoing presence of Theileria organisms within erythrocytes, although the anemia had marginally improved (hematocrit 0.18 L/L and hemoglobin 60 g/L). The cow was submitted to AHL-Kemptville for postmortem examination. Significant gross postmortem lesions consisted of marked edema and diffuse enlargement of the pelvic, hepatic, and peri-ruminal lymph nodes, mildly watery pale blood, and mild icterus of internal fat. Lameness was attributed to localized fibrinopurulent myositis of left thigh muscles, and periarthritis and arthritis of the left femorotibial joint. Histology of the enlarged lymph nodes showed cortical lymphoid hyperplasia, with germinal centers. In one node, medullary cords were populated predominantly by small lymphocytes, with scattered foci of extramedullary hematopoiesis. Lymphocytic schizonts were not seen in the lymph nodes, and are reported as uncommon in cows infected with T. buffeli. Bone marrow taken at postmortem was autolysed, and only adipose tissue with mild intercellular hemorrhage and scattered foci of hematopoiesis were identified.
This is the first documented identification of this parasite in cattle in Canada. Neither the affected cow nor her clinically unaffected herd mates had ever travelled outside of Ontario. Theileria are tick transmitted, protozoal erythroparasites of ruminants. The tick species associated with T. buffeli transmission has not been identified. The role of wildlife such as white-tailed deer as a possible parasite reservoir is also unclear. Although this cow had been on pasture, it is not known how she became infected.
Theileria buffeli has been reported previously in individual cows in the United States (Kansas 1950, Texas 1975, Missouri 2000, Michigan 2002 and 2014). T. buffeli has been considered non-pathogenic or less pathogenic than other Theileria spp. such as T. parva and T. annulata which are the agents of the rapidly fatal East Coast fever in Africa, and tropical theileriosis in the Mediterranean and Asia, respectively. Unlike the intralymphocytic schizonts which are considered the major pathogenic stage for T. parva and which also play a role in T. annulata infections, the intraerythrocytic piroplasms are the major pathogenic state for T. buffeli. T. buffeli has been identified both in asymptomatic cattle, as well as those with clinical evidence of anemia. The pathogenesis of anemia has not been clearly established and may be multifaceted, involving both immune-mediated mechanisms as well as erythrocyte fragmentation and damage by proteases and oxygen radicals. Lymphoid hyperplasia and lymphoma have been previously identified with bovine theileriosis. It is known that intra-lymphocytic theilerial parasites can transform cells, and lead to the clonal proliferation of lymphocytes, although it is unclear if this occurs with T. buffeli infections.
Currently, Theileria can only be detected by examination of peripheral blood smears. Therefore evaluation of a well-prepared blood smear is strongly recommended for all anemic ruminants.
Theileriosis is on the federal and provincial lists of immediately notifiable diseases, and therefore this case was reported to OMAFRA and the CFIA.

Figure 1. Intraeryrthrocytic piroplasms of Theileria buffeli (arrows).
HORSES
Ontario Racing Commission Death Registry: 2014 postmortem summary
The Ontario Racing Commission (ORC) has a long-established record and takes pride in its proactive approach to advancing the welfare of the racehorse and the safety of the participant. In 2003, Ontario became one of the first North American racing jurisdictions to require mandatory reporting of racehorse deaths, in order to monitor, research, and improve our knowledge of why these tragic events occur. The ORC Death Registry continues to provide excellent data regarding the causes of morbidity and mortality in racehorses in this province. Summaries of postmortem submissions to the AHL under this program and diagnoses by body system are provided in the following tables.
Table 1. Breed distribution of ORC Death Registry submissions to the AHL, 2003-2014
|
Breed /Year |
Standardbred |
Thoroughbred |
Quarter Horse |
Total |
|
2003 |
67 (54%) |
58 (46%) |
0 |
125 |
|
2004 |
82 (58%) |
60 (42%) |
0 |
142 |
|
2005 |
59 (54%) |
51 (46%) |
0 |
110 |
|
2006 |
58 (54%) |
47 (44%) |
2 (2%) |
107 |
|
2007 |
66 (54%) |
53(43%) |
3(3%) |
122 |
|
2008 |
27 (53%) |
24(47%) |
0 |
51 |
|
2009 |
28 (62%) |
16 (36%) |
1 (2%) |
45 |
|
2010 |
22 (69%) |
8 (25%) |
2 (6%) |
32 |
|
2011 |
24 (52%) |
18 (39%) |
4 (9%) |
46 |
|
2012 |
20 (59%) |
14 (41%) |
0 |
34 |
|
2013 |
19 (40%) |
27 (56%) |
2 (4%) |
48 |
|
2014 |
21 (41%) |
23 (45%) |
7 (14%) |
51 |
Table 2. Postmortem diagnoses of ORC Death Registry submissions by body system, 2003-2014.
|
Diagnosis by body system: |
2003 |
2004 |
2005 |
2006 |
2007 |
2008 |
2009 |
2010 |
2011 |
2012 |
2013 |
2014
|
|
Fracture / limbs |
53 (42%) |
69 (49%) |
48 (44%) |
42 (39%) |
54 (44%) |
16 (31%) |
4 (9%) |
9 (28%) |
6(13%) |
2 (6%) |
23 (48%) |
23 (45%) |
|
Fracture / other |
10 |
4 |
7 |
13 |
10 |
5 |
0 |
3 |
6 |
2 |
2 |
7 |
|
Non-fracture musculoskeletal |
8 |
6 |
6 |
8 |
6 |
5 |
2 |
3 |
1 |
0 |
3 |
4 |
|
Gastrointestinal |
15 |
19 |
17 |
16 |
18 |
5 |
4 |
7 |
5 |
6 |
4 |
6 |
|
Respiratory (including EIPH) |
21 |
17 |
9 |
11 |
16 |
9 |
21 |
6 |
9 |
7 |
4 |
5 |
|
Cardiovascular |
5 |
6 |
5 |
5 |
2 |
4 |
6 |
2 |
4 |
1 |
7 |
3 |
|
CNS |
6 |
11 |
7 |
4 |
1 |
1 |
2 |
0 |
5 |
4 |
3 |
0 |
|
Integumentary |
0 |
0 |
1 |
2 |
2 |
1 |
1 |
0 |
0 |
0 |
0 |
0 |
|
Renal |
0 |
2 |
0 |
0 |
2 |
0 |
1 |
0 |
0 |
0 |
0 |
0 |
|
Hematopoietic |
2 |
1 |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
Other / whole body conditions (e.g., septicemia) |
1 |
7 |
5 |
2 |
9 |
0 |
4 |
0 |
6 |
6 |
2 |
1 |
|
Cause of death undetermined |
4 (3.2%) |
0 (0%) |
4 (3.6%) |
4 (3.7%) |
2 (1.6%) |
5 (9.8%) |
0 (0%) |
2 (6%) |
4 (9%) |
6 (18%) |
0 (0%) |
2 (4%) |
Total |
125 |
142 |
110 |
107 |
122 |
51 |
45 |
32 |
46 |
34 |
48 |
51 |
Table 3. Musculoskeletal injuries in ORC Death Registry submissions by breed and anatomic site, 2014.
|
Lesion |
TB |
SB |
QH |
Total |
|
P1 fracture - LF |
0 |
2 |
0 |
2 |
|
P1 fracture - RF |
1 |
1 |
0 |
2 |
|
P1 fracture - LH |
2 |
1 |
0 |
3 |
|
P1 fracture - RH |
0 |
2 |
0 |
2 |
|
Carpal fracture - R |
2 |
0 |
1 |
3 |
|
Proximal sesamoid fracture – LF medial and lateral sesamoids |
2 |
1 |
0 |
3 |
|
Fetlock failure - LF |
1 |
0 |
0 |
1 |
|
Fetlock failure - RF |
2 |
0 |
0 |
2 |
|
Humerus fracture - L |
0 |
2 |
0 |
2 |
|
Metatarsal III fracture - L |
0 |
2 |
0 |
2 |
|
Tibia fracture - L |
1 |
0 |
0 |
1 |
|
Pelvis fracture |
1 |
0 |
0 |
1 |
|
Rib fracture |
0 |
1 |
0 |
1 |
|
Vertebral fracture |
0 |
1 |
1 |
2 |
|
Skull fracture |
3 |
0 |
0 |
3 |
|
Sacroiliac subluxation |
0 |
0 |
1 |
1 |
|
Flexor tendon laceration – RF |
0 |
1 |
0 |
1 |
|
Carpus DJD - bilateral |
1 |
0 |
0 |
1 |
|
Multiple soft tissue lacerations |
0 |
0 |
1 |
1 |
|
Total by breed |
16 |
14 |
4 |
34 |
Table 4. Non-musculoskeletal diagnoses in ORC Death Registry submissions, 2014.
|
Gastrointestinal: |
Hemorrhagic enterocolitis associated with Clostridium perfringens (1) |
|
|
Colitis associated with Clostridium difficile (1) |
|
|
Typhlitis / typhlocolitis / colitis, etiology undetermined (3) |
|
|
Large colon torsion (1) |
|
Respiratory: |
Exercise-induced pulmonary hemorrhage (EIPH) (2) |
|
|
Pulmonary congestion and edema, suspect heart failure (1) |
|
|
Laryngotracheal evulsion secondary to trauma (1) |
|
|
Tracheal malformation (1) |
|
Cardiovascular: |
Hemoabdomen, with concurrent EIPH (2) |
|
|
Hemoabdomen and retroperitoneal hematoma (1) |
|
Other / whole body conditions: |
Hypothyroidism (1) |
Potomac horse fever (Neorickettsia risticii) infection in 2 aborted Ontario foals
Andrew Vince, Andrew Brooks, Hugh Cai
In December and January of 2014-2015, we received 2 aborted foals, the first at 5 months gestation, the second at 7 months gestation – one from eastern Ontario, one from southwestern Ontario. In the first foal, there was 100 mL blood-tinged free fluid in the peritoneal cavity and diffuse firmness of both lungs. The second foal had generalized icterus; multifocal hemorrhages in the skin, skeletal muscle, and small intestinal mucosa; subtle enlargement and pallor of the liver; and large quantities of fluid in the small intestine and colon. Tissues from both animals were negative by PCR for equine herpesvirus-1 (EHV-1). No significant pathogens were isolated on bacterial culture of lung, stomach content, or placenta (as available).
Despite divergent gross lesions, histologic lesions were similar in both cases, and included hepatitis, myocarditis, thymic necrosis/thymitis, and most distinctively erosive enteritis (an unusual lesion in an aborted foal). The first foal also had a distinctive vasculitis in placenta, lung, and thymus. More uncommon differential diagnoses for equine abortion were discussed, including leptospirosis, equine viral arteritis (EVA), and Potomac horse fever (PHF). The first fetus was negative for EVA (PCR on lung) and leptospirosis (immunohistochemistry on various tissues). PCR tests of spleen and liver for PHF was positive in both foals. This result strongly implicated PHF as causal in both abortions.
Neorickettsia risticii is the causal organism underlying Potomac horse fever, a disease characterized by acute enterocolitis in horses first identified in 1979. It is principally associated with disease in spring, summer, and fall, and is most common in farms bordering rivers. This is a very infrequently identified cause of equine abortion in Ontario.
Abortion typically occurs months after infection and clinical disease in the mare, and is associated with fetal infection resulting in enterocolitis, hepatitis, myocarditis, placentitis, and variable hyperplasia or depletion of lymphoid organs. The gross and histologic appearance may initially resemble EHV-1 (though without classic microscopic herpesviral inclusions), and PHF should be considered a differential diagnosis for abortion in such cases if EHV-1 PCR/IHC testing is negative, regardless of season. PCR testing of tissues (usually lung, liver, spleen, commonly collected for EHV-1 PCR in such cases) is available at the AHL.
SWINE
The Swine OAHN (Ontario Animal Health Network)
Melanie Barham
The Ontario Animal Health Network is up and running in the swine sector!
What is OAHN? OAHN is a new way for Ontario commodity groups to tackle important disease issues in their sector, collaborate with other industries, and access valuable resources. Each sector will have an “Expert Network” comprising an AHL, OVC, and OMAFRA species specialist and up to 3 private practitioners. The Expert Network will meet regularly to discuss pertinent diseases and issues affecting the sector. Laboratory data will be discussed, together with the results of a quarterly veterinary survey. The network’s focus is on identifying trends and actionable items for the industry, and will work together with producer groups. Networks will also participate in cross-species information sharing, and the OAHN plan will be able to link in with other provinces and a national program as these initiatives are developed.

Networks currently in operation:
Fish, poultry, small ruminants, swine.
Under development:
Bees, bovine, companion animals, equine, fur-bearing/alternative, wildlife.
Swine OAHN
* OAHN is patterned on CSHIN (Canadian Swine Health Information Network), and will serve as the Ontario node if CSHIN is funded and continues.
* The second swine network teleconference was held January 29th to discuss Oct/Nov/Dec 2014 clinical impression survey information. The AHL and Gallant Custom Laboratories provided lab data for the quarter.
* A veterinary report and a producer report were published and distributed. The producer report focus was post-weaning colibacillosis.
* OAHN data is being shared with CSHIN’s national program.
* Ontario Pork and OSHAB are close advisors for this network and we look forward to a continued close relationship with producer groups. The full reports can be obtained by emailing barhamm@uoguelph.ca .
* Ongoing surveillance testing for PED has been funded through OAHN.
* Check out our OAHN podcast about neonatal piglet diarrhea!
OAHN Podcasts - Our podcasts are available in iTunes, so you can access them via any Apple device and subscribe so you never miss an episode. As always, they are also available on our podbean site: barhamm.podbean.com .
Social media - Our Facebook and Twitter (@OntAnHealthNet) feeds offer up to date disease notifications, as well as news stories and information for you to repost to your clinic website or Facebook/Twitter feeds if you so choose.
Website - We are designing a new website for the OAHN program, launching this spring.
Farm press - The OAHN program was also featured in an Ontario Farmer article.
Questions? Comments? Would you like a copy of the reports? Do you want to be included on surveys or mailing lists? Contact Dr. Melanie Barham at (519) 824-4120 x53364 or barhamm@uoguelph.ca.
Website: OAHN Website
Podcasts: OAHN Podcasts
AVIAN/FUR/EXOTIC SPECIES
Yellow fungus disease in bearded dragons
Marina Brash, Durda Slavic, Hugh Cai, Pat Bell-Rogers, Megan MacAlpine
In the last quarter of 2014, the AHL received numerous submissions of primarily juvenile bearded dragons with histories of lethargy and death often following the development of skin lesions. Gross skin lesions ranged from yellow to grey/brown ulcerated to thickened, roughened and scaly patches involving the face, head, neck, abdomen, legs and tail (Fig. 1) with splitting of the skin reported in some chronic cases.
Histologically, variable numbers of fungal hyphae were on the surface and within a variably thickened hyperkeratotic layer of epidermis, and the underlying epithelium was eroded/ulcerated or hyperplastic. Often the dermis was expanded, hypercellular, fibrotic, and infiltrated by nodules or sheets of macrophages and multinucleated giant cells, often containing fungal hyphae. Occasionally the nodules had central cores of eosinophilic necrotic debris. Granulomas were also within the underlying skeletal muscle, connective tissue between muscle bundles, and adjacent to resorbing bone. Organs including lung and liver were less frequently affected (Fig. 2).
Fresh skin samples were submitted for fungal culture, and wet mount microscopic examination, which revealed a large number of hyphae confirming histological observations. Fungal cultures yielded Chrysosporium anamorph of Nannizziopsis vriesii (CANV) which was confirmed by the mycology laboratory at the Public Health Agency of Canada and also by 18S rRNA gene sequencing at the AHL.
In the past, it was widely accepted that CANV and a variety of Chrysosporium spp. cause yellow fungus disease in reptiles. Advanced molecular characterization of over 40 isolates from reptiles showed that they had been phylogenetically misclassified. Isolates from reptile dermatitis cases belong to 3 different fungal genera, namely Nannizziopsis, Paranannizziopsis, and Ophidiomyces. These isolates may have species predilections, with O. ophiodiicola causing disease in snakes only, whereas N. guarroi primarily affects bearded dragons.
There are slight morphological differences among the isolates belonging to these genera. Since, at present, molecular characterization is time consuming, expensive, and likely does not affect the clinical outcome, AHL reports fungal isolates from reptile dermatitis cases as CANV complex which currently encompasses all 3 fungal genera noted above.
|
|
|
|
Figure 1 a. Orange bearded dragon with grey/brown irregularly thickened and crusted skin of the caudal back, R leg, and proximal tail. The R leg and tail are swollen, and the tail is shortened with a dark crust on the tip. b. Ventrum of head of a bearded dragon; generalized yellow discoloration of the skin and yellow crusts of exudate at the neck. c. Skin of the ventral abdomen is slightly thickened and yellow with scattered small brown foci. (Photo courtesy of Dr. Rick Axelson, The Links Road Animal & Bird Clinic). |
Figure 2 a. Surface keratin contains large numbers of fungal hyphae (*). The underlying epidermis is hyperplastic (200X, H & E). b. Multiple nodules and clusters of macrophages and giant cells (M), often containing fungal hyphae (see inset (400X, H & E) are within the expanded, fibrotic dermis and infiltrating into the underlying skeletal muscle (S) (100X, H & E). c, d. Granulomas occasionally containing fungal hyphae (*) within liver (c) and lung (d). Exudate (E) is accumulating in faveolar lumens |
COMPANION ANIMALS
Leptospiral nephritis in a Shih Tzu dog
Margaret Stalker, Jessika Bronsoiler
A 4-year-old spayed female Shih Tzu living in Brantford, Ontario, was presented with a history of partial anorexia of one-month duration. Clinical workup had been previously refused, and the dog had been treated symptomatically with famotidine. On presentation, the dog was dehydrated and severely azotemic. The dog was hospitalized, treated with intravenous fluids, antibiotics, and antiemetics without significant improvement and the owner elected euthanasia. Sections of kidney collected at postmortem were submitted for histologic examination at the AHL.
Histology revealed extensive renal interstitial inflammatory infiltrates (Fig. 1), with accompanying mild interstitial fibrosis and tubular atrophy. The inflammatory infiltrate was of mixed cell types, with nodular aggregates as well as more diffuse infiltrates of lymphocytes and plasma cells, scattered macrophages, and infiltrates of neutrophils within the cortical interstitium. There was edema and patchy irregular foci of fibrin exudation and neutrophilic infiltrates within the medullary interstitium. The unusually severe and active inflammation of this lymphoplasmacytic and suppurative tubulointerstitial nephritis prompted us to perform immunohistochemistry, looking for Leptospira interrogans. Widespread positive staining was present (Fig. 2), including extensive intense linear and granular staining (the latter representing degraded bacteria) within tubular lumens, tubular epithelial cells, and also within macrophages in the interstitium. Staining with Warthin-Starry silver stains also highlighted intact organisms morphologically compatible with leptospires in renal tubular lumens (Fig. 3).
Naturally occurring infection with Leptospira spp. is relatively common in various wildlife populations in southern Ontario, principally raccoons and striped skunks. Infection in these species is apparently self-limiting and asymptomatic, with the host recovering from the initial stages of the disease while potentially remaining as a reservoir capable of continued urinary shedding of infectious bacteria. Epidemiologic studies of canine leptospirosis demonstrate increased risk of infection in middle-aged, intact male hounds, herding, working or mixed-breed dogs, presumably associated with increased outdoor activity of these breeds. However, the presence of large urban populations of reservoir wildlife species such as raccoons increases the chance of exposure of urban dogs with access to the outdoors as well.
The severe lymphoplasmacytic and neutrophilic tubulointerstitial nephritis seen in this case is a compatible lesion of subacute infection in dogs, although acute infections may cause significant hepatorenal dysfunction (with depression, anorexia, vomiting, diarrhea, icterus, dehydration, severe azotemia) with only minimal, subtle histologic lesions in the kidney and liver. Antemortem diagnosis has relied on detection of high serum antibody titers using the microscopic agglutination test (MAT), although peracute/acute infections may have low titers. PCR testing of blood or urine, and immunohistochemistry on renal biopsies may be useful for detection of early infections and confirmation of infection in some instances.
|
|
|
|
|
Figure 1. Lymphoplasmacytic, histiocytic and suppurative tubulointerstitial nephritis in a dog (H&E).
|
Figure 2. Positive immunostaining for Leptospira interrogans in tubular lumens, tubular epithelial cells and interstitial macrophages.
|
Figure 3. Aggregates of typical curving spirochete bacteria in tubular lumens (GMS silver stain).
|