AHL Newsletter, December 2016
For a pdf copy of AHL Newsletter, December 2016 click here
AHL Holiday Hours, 2016/17
Season’s Greetings from the staff of the Animal Health Laboratory
Except for closure on Christmas Day, Dec 25, the AHL is open every day with limited services; the U of Guelph is officially closed Friday Dec 23 through Monday Jan 2, 2017. Guelph and Kemptville drop box and/or refrigerator are available 365/24/7 for specimen drop off. Guelph - Usual Saturday services include: specimen receiving, emergency mammalian autopsies, full bacteriology set up, as well as clinical pathology testing. Statutory holiday services and usual Sunday services include: specimen receiving, emergency mammalian PMs, and bacteriology set up. For full details, please see our website—ahl.uoguelph.ca

Dr. Durda Slavic - Bio-Mic Award for Excellence in Diagnostic Veterinary Microbiology
We’re proud to announce that Durda Slavic, DVM, MSc, PhD, AHL veterinary bacteriologist since 2004, is the 2016 recipient of the Bio-Mic Award for Excellence in Diagnostic Microbiology presented at the annual meeting of the American Association of Veterinary Laboratory Diagnosticians, held in Greensboro, North Carolina, Oct. 13-17, 2016. The award letter from Dr. Pat Halbur, President of AAVLD, stated: “Your commitment to advancing diagnostic microbiology and support of AAVLD provide a foundation for the current and future success of this specialty. Your efforts have left an indelible mark for future diagnosticians and leaders of AAVLD.”
Congratulations Durda!

EIA electronic submissions are now being accepted
AHL has just finished participating in a trial with CFIA and GlobalVetLink (EquusLink), and was the pilot laboratory in Canada for this exciting new service. We thank the CFIA for taking up this initiative.
You can learn more about EquusLINK at: https://www.globalvetlink.com/products/equuslink/
Advantages of this product include:
- Faster form completion, including immediate updating of information for correction of omissions.
- Streamlined submission to the lab, including embedded color photographs of horses rather than hand drawings.
- Electronic reporting of results, and streamlined transmission of test results to CFIA.
- All results stored for immediate retrieval and immediate use (no more mailing results).
We have found this tool to be beneficial in streamlining EIA submissions, and we look forward to offering this solution to our clients! Traditional EIA submission forms will of course still be accepted.
Contact us at ahlinfo@uoguelph.ca or (519) 824-4120 ext. 54530
AHL flexible scope update
Liz King
The AHL is accredited for specific tests listed on our SCC scope of accreditation or “fixed scope”. Because there can be a lag in applying for and adding a test to a fixed scope, the AHL became accredited in June 2014 for veterinary laboratory testing techniques (flexible scope). See AHL’s SCC scope, both fixed and accredited techniques, at http://palcan.scc.ca/specs/pdf/826_e.pdf.
AHL’s flexible scope means that the AHL is accredited by the Standards Council of Canada (SCC) to ISO/IEC 17025 for the following veterinary (medical) techniques:
1. Culture detection of microorganisms
2. Inorganic analysis by inductively coupled plasma spectroscopy (ICP)
3. Enzyme linked immunosorbent assay (ELISA)
4. Agglutination
5. Polymerase chain reaction (PCR)
The AHL adds specific methods under each accredited technique when 2 criteria are met:
There is a demonstrated need for accreditation for a method for which AHL is not already accredited.
AHL’s quality assurance (QA) has verified that the test method meets all of SCC’s accreditation requirements.
In addition to QA inspections, SCC inspects AHL’s flexible scope process and the list of flexible scope methods every 2 years.
AHL currently has 11 different methods, each containing multiple agents/elements, on our flexible scope. The most recent additions to our scope are:
ELISA method V-002 for Coxiella burnetii (Q fever)
PCR method MOL-262 for Echinococcus multilocularis
Contact us at ahlinfo@uoguelph.ca if you would like a copy of AHL’s flexible scope. AHL
AHL Newsletter
December, 2016 - Volume 20, Number 4
Editor: Grant Maxie, DVM, PhD, Diplomate ACVP
Editorial Assistants: Helen Oliver, April Nejedly
The AHL Newsletter is published quarterly (March, June, September, December) by the Animal Health Laboratory, Laboratory Services Division, University of Guelph.
Its mission is to inform AHL clients and partners about AHL current activities, and laboratory-based animal disease events and disease trends. All material is copyright 2016. Ideas and opinions expressed herein do not necessarily reflect the opinions of the University or the Editor.
Articles may be reprinted with the permission of the editor and with appropriate credit given to the AHL Newsletter.
Mailing address & contact information:
Animal Health Laboratory
Laboratory Services Division, University of Guelph
Box 3612, Guelph, Ontario, Canada N1H 6R8
Phone: (519) 824-4120 ext. 54538; fax: (519) 821-8072
To receive an electronic copy of this Newsletter, please send your email address to us at holiver@uoguelph.ca
ISSN 1481-7179
Canada Post Publications number - 40064673
Contributors to this issue - from the Animal Health Laboratory:
Melanie Barham, DVM, PMP
Marina Brash, DVM, DVSc, Diplomate ACVP
Andrew Brooks, DVM, PhD, Diplomate ACVP
Hugh Cai, DVM, MSc, DVSc
Michael Deane
Josepha DeLay, DVM, DVSc, Diplomate ACVP
Jim Fairles, DVM, MBA
Elizabeth King, MSc
Davor Ojkic, DVM, PhD
Felipe Reggeti, DVM, PhD, Diplomate ACVP
Nick Schrier, MSc
Jan Shapiro, DVM, DipEqSurg, DipPath
Durda Slavic, DVM, PhD
Maria Spinato, DVM, DVSc, Diplomate ACVP
Andrew Vince, DVM, DVSc, Diplomate ACVP
Other contributors:
Jocelyn Jansen, DVM, DVSc; OMAFRA, Elora, ON.
Brent Jones, DVM, Stratford, ON.
Kevin Vilaca, DVM, MSc, St. Clements, ON.
Andria Jones-Bitton, DVM, PhD; Paula Menzies, DVM, MPVM DipECSRHM, Population Medicine; Andrew Peregrine, BVMS, PhD, DVM, DipEVPC, DipACVM, Pathobiology, OVC, Guelph, ON.
Our continued thanks to all of the non-author AHL clerical, technical, and professional staff who contribute to the generation of results reported in the AHL Newsletter.
OAHN Update December 2016
 Annual meeting: We are also excited to have had a successful annual meeting of the Disease Surveillance Plan, which took place as a workshop on October 5 at the Holiday Inn in Guelph. This workshop was beneficial for the program, as it brought together private veterinarians, government employees, professors, researchers, pathologists, and industry members to discuss how best OAHN can be utilized to help with animal health in Ontario. You can view the final report on OAHN.ca, which includes the agenda, attendees, the executive summary, and network-specific responses to all of the questions posed during the day.
Annual meeting: We are also excited to have had a successful annual meeting of the Disease Surveillance Plan, which took place as a workshop on October 5 at the Holiday Inn in Guelph. This workshop was beneficial for the program, as it brought together private veterinarians, government employees, professors, researchers, pathologists, and industry members to discuss how best OAHN can be utilized to help with animal health in Ontario. You can view the final report on OAHN.ca, which includes the agenda, attendees, the executive summary, and network-specific responses to all of the questions posed during the day.
New podcasts!
- Lyme disease and ticks in humans and companion animals with Dr. Michelle Evason, Curtis Russell (PHO)
- 4 part series on Salmonella Dublin, including pathology, clinical review, Salmonella Dublin in Quebec, and the current surveillance of S. Dublin being done through the OAHN Bovine Network’s project.
Do you ask your clients for their Premises IDs? Check out how fast and easy it is to do by registering your vet clinic: https://www.ontarioppr.com/home_en.html

Find out about recent bee health news here in the 1st OAHN bee report!

The small ruminant network met in October. It was a quiet quarter for sheep and goat diseases; stay tuned for the report.

The OAHN Bovine Network released its latest reports in November, discussing the quarterly survey, calf health surveillance project, and more. The bovine network also published a four part series on Salmonella Dublin, which you can access at http://oahn.podbean.com/. If you want more information or wish to enroll in the network’s surveillance project, email Dr. Ann Godkin at ann.godkin@ontario.ca.

The OAHN fish network released its second quarterly report in September, including an aquatic veterinary services disease summary, provincial and federal updates, and information on the OAHN research project.

Recent producer and vet reports can be found here. The swine network has also created a Senecavirus A screening procedure for producers, which is available on the veterinary side of OAHN.ca.

On these calls we discuss interesting cases with experts, and we have a listserv to trade case and treatment ideas. Email oahn@uoguelph.ca to join.

The Q4 2016 conference call was held in November. OAHN data is also being collated for use in the upcoming producer updates across Ontario.
Small flock corner:
OAHN held its first small flock conference call on September 14th. Interesting cases in backyard flocks were discussed, with veterinarians providing expert advice. A video recording of the call with slideshows is available on the veterinary side or our site (must be signed in). You can still sign up for the Small Flock Listserv by emailing oahn@uoguelph.ca .

The equine network released its Q3 veterinary and owner reports in November. The reports covered the following issues: muscle biopsies, recent Lawsonia cases, and survey results. OAHN also participated in an equine disease surveillance workshop hosted by CAHSS and Equestrian Canada in Toronto.

We now have an OAHN wildlife network! The group includes OMNRF, OMAFRA, CWHC, OVC, and public health, and will focus on collaborating on emerging issues in the Ontario wildlife population. The next meeting in November. Stay tuned!

The OAHN companion animal network released its quarterly report in November. Main discussion items included heartworm, travel-related diseases, and ticks and giardiasis.
The Companion Animal Network also published a new infographic on “Using the Best Medicine and Reducing Antibiotic Use,” which you can access here.
RUMINANTS
Bovine astrovirus – emergence of a novel viral encephalitis in cattle
Maria Spinato, Andrew Vince, Hugh Cai, Davor Ojkic
In May (steer A) and June (steer B) 2016, the AHL received fresh and formalin-fixed samples of brain from 2 steers located in separate feedlots, each comprised of 25-50 animals. Both steers had been found dead after exhibiting neurologic signs for several days. In steer B, tonic-clonic seizures were elicited by stimulation, and repetitive licking and chewing movements were also described. Prior to laboratory testing at the AHL, brain samples from both animals were submitted for Rabies virus fluorescent antibody testing by the Canadian Food Inspection Agency; both tested negative.
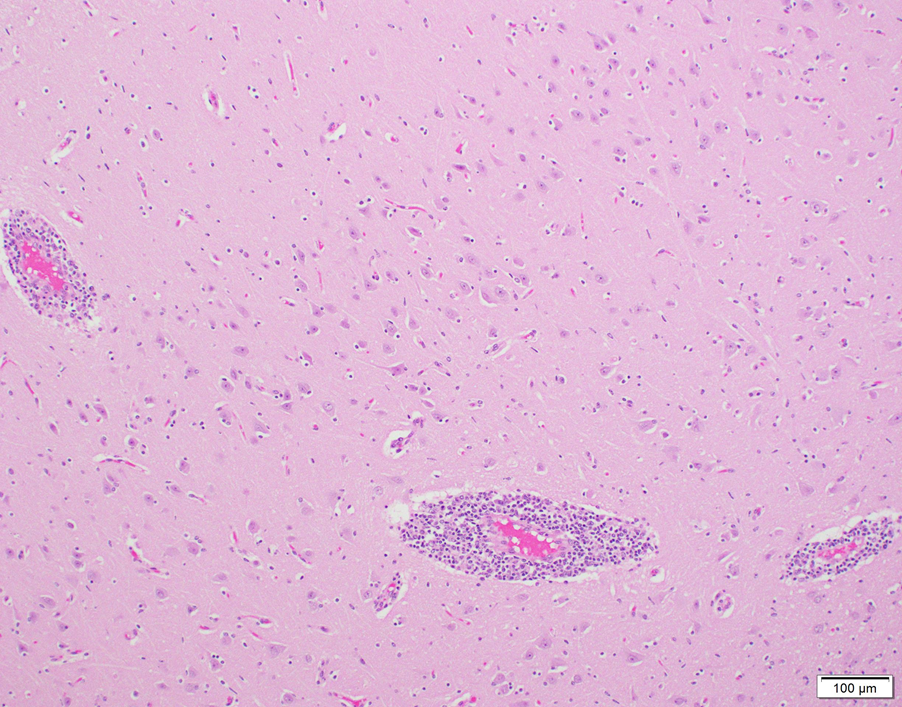
Histologic evaluation of brain revealed generalized, broad perivascular cellular cuffs extending from the cerebral cortex into the brainstem. Leukocytic cuffs expanding Virchow-Robin spaces were comprised of 3-8 layers of lymphocytes with occasional admixed histiocytes, rare neutrophils, and plasma cells (Fig. 1). Mild focal microgliosis and rare axonal spheroids were also observed in some sections. Regional nonsuppurative meningitis was present; vasculitis was not observed.
Based on the histologic lesions and the negative rabies test result, listerial meningoencephalitis and viral infections such as Bovine herpesvirus 1 and Ovine herpesvirus 2 (OvHV-2, Sheep-associated malignant catarrhal fever of cattle virus), were considered to be possible etiologies. Immunohistochemical staining for Listeria monocytogenes in paraffin-embedded sections of brain was negative in both cases, as were PCR tests for BoHV-1 and OvHV-2 in fresh sections of brain (OvHV-2 PCR performed by Prairie Diagnostic Services, Saskatoon, SK).
Idiopathic nonsuppurative meningoencephalitis is an occasional default final diagnosis in cattle with neurologic disease, given that a cause cannot be identified despite testing for a wide range of viruses, bacteria, and protozoa. Because of recent reports from Europe and the US of a novel astrovirus detected in cases of bovine nonsuppurative encephalitis, samples of brain from these 2 steers were tested for astrovirus by PCR. A RT-PCR specific for mammalian astrovirus1 was positive in both cases. The 385 bp PCR products were sequenced and demonstrated 95% homology with the US bovine astrovirus NeuroS12, and 93% homology with the Swiss bovine astrovirus CH13/NeuroS1 isolates3.
To our knowledge, this is the first confirmed report of bovine astrovirus-associated encephalitis in Canadian cattle.
It is suspected that neurotropic astroviruses have been circulating in global cattle populations for several decades. Swiss researchers who performed in situ hybridization on paraffin-embedded brain from cattle diagnosed with idiopathic encephalitis found astrovirus RNA in a high proportion of samples. However, viral association with histologic lesions was inconsistent, and the authors could not conclusively rule out a bystander role for this virus.4 Infection studies would assist in confirming the neurovirulent potential of bovine astrovirus, but have not been performed to date because the virus has yet to be isolated in cell culture.
Further work is in progress at the AHL to examine the association of bovine astrovirus and cases of idiopathic nonsuppurative encephalitis in Ontario cattle. AHL
References
- Mittelholzer C, et al. Molecular characterization of a novel astrovirus associated with disease in mink. J Gen Virol 2003;4:3087-3094.
- Li L, et al. Divergent astrovirus associated with neurologic disease in cattle. Emerg Infect Dis 2013;19:1385-1392.
- Bouzalas IG, et al. Full-genome based molecular characterization of encephalitis-associated bovine astrovirus. Infect Genet Evol 2016;44:162-168.
- Selimovic-Hamza ,et al. Detection of astrovirus in historical cases of European sporadic bovine encephalitis, Switzerland
 -ansi-language:en-CA;mso-ligatures:none'>
-ansi-language:en-CA;mso-ligatures:none'>
Figure 1. Non-suppurative perivascular cuffing in bovine brain in a case of astrovirus-associated encephalitis
Sign up for the Adult Small Ruminant Mortality Project!
Maria Spinato, Jocelyn Jansen, Paula Menzies, Andria Jones-Bitton
The AHL, OVC, and OMAFRA have received funding to conduct a joint study investigating adult small ruminant mortalities. Sick or dead adult sheep and goats are rarely sent to a laboratory for complete postmortem examination, and veterinarians infrequently perform postmortems on farm. However, there is value in knowing why an adult small ruminant died, especially during the current rapid expansion of small ruminant operations in Ontario.
The objectives of this study are:
- To determine why adult sheep and goats are dying on-farm.
- To determine if a web-based postmortem information and case submission system can be used to increase the usefulness of on-farm postmortems.
- To determine if better disease diagnoses can increase discussions between producers and their vets, so that they can create sound flock/herd health and biosecurity plans.
The project will fund:
- tissue collection kit and shipping fee,
- AHL laboratory test fees,
- compensation for veterinarian performing the on-farm postmortem.
In return, the veterinarian will submit:
- a complete set of fresh and formalin-fixed tissues, as per project protocol,
- a set of 6 postmortem photographs that are uploaded to the website,
- postmortem submission form with all required information, also uploaded to the website.
Funding support is being provided by the OMAFRA-University of Guelph Partnership KTT Program and the Ontario Animal Health Network. Thanks to the generosity of these agencies, ~170 postmortems of adult sheep and goats will be performed on farm over the next 12-16 months. A standardized diagnostic test panel has been developed to check for the diseases of greatest importance to the small ruminant industry.
Interested in participating?
For more information, contact the project administrators at sr.mort@uoguelph.ca or log onto the project web-site at https://www.uoguelph.ca/srmort/index.php/veterinarian/ AHL
SWINE
Mycoplasmal arthritis in swine
Josepha DeLay, Jim Fairles, Hugh Cai
Mycoplasma hyorhinis and M. hyosynoviae can contribute to infectious arthritis in swine. M. hyorhinis typically causes disease in pigs <10 weeks of age, and may lead to arthritis alone or, more commonly, in conjunction with polyserositis. In contrast, M. hyosynoviae usually causes lameness in pigs >10 weeks old, although there may be overlap in these clinical scenarios. Some practitioners have identified an increased prevalence of arthritis caused by Mycoplasma spp. in North American swine. This may, in part, reflect the increased sensitivity of new diagnostic tests using PCR techniques. Clinically, a diagnosis of mycoplasmal arthritis is often made on the basis of response to therapy. At the AHL, an average of 3 cases of mycoplasmal arthritis were confirmed annually from 2012 to 2016, most commonly M. hyosynoviae arthritis in feeder or finisher pigs.
Pigs are infected with M. hyorhinis and M. hyosynoviae from sows, or from infected herdmates at nursery age. Both may be commensal organisms, with localization of M. hyorhinis in the upper respiratory tract, and persistence of M. hyosynoviae in tonsil. Bacteremia precipitated by stress may lead to joint localization, arthritis, and clinical lameness. Any joint, and single or multiple joints, may be affected in an individual pig.
PCR is the method of choice for detection of both M. hyosynoviae and M. hyorhinis in joint fluid aspirates (preferred) or swabs. Presence of either organism in joints does not always lead to arthritis and because of this, PCR results must be correlated with histologic evidence of arthritis. Selection of appropriate pigs for postmortem exam and sampling is imperative in order to confirm mycoplasmal arthritis. Multiple (2-3) acutely affected, untreated animals should be identified clinically and euthanized for postmortem examination and sampling. Joints may not be obviously swollen in acutely affected animals, and clinical observation and identification of pigs that are reluctant to stand or overtly lame is very important in selecting optimal candidates for sampling. Chronically lame pigs, or pigs that are found dead, are not useful for diagnostic testing. In these circumstances, the primary pathogen responsible for infectious arthritis may have been eliminated and only secondary, opportunistic pathogens may be present.
Optimally, entire carcasses should be submitted to the diagnostic laboratory for evaluation of the cause of lameness. Many practitioners prefer to submit only multiple intact limbs from affected pigs for examination sampling, however more information may be gained if the entire body is examined. If on-farm sampling is undertaken, it is important to be organized and to maintain aseptic conditions for tissue collection. After removing skin over the joints, fluid from each joint should be aseptically aspirated and examined. Pigs with mycoplasmal arthritis usually have an increased amount of red-tinged, variably cloudy joint fluid. For each joint, the joint capsule should then be incised, and swabs taken from the synovial surface for bacterial culture. Multiple synovial samples are fixed in formalin for histologic examination, and an unfixed synovial sample is held frozen for additional PCR testing, if needed. Once opened, all joints should be examined for lesions of osteochondrosis (OCD), fractures, periarticular abscesses, or other conditions that may be contributing to lameness. Long bones should be examined grossly and histologically for evidence of metabolic bone disease. In lame animals that lack obvious joint lesions, examination of pelvis, vertebrae and spinal cord, and brain is necessary to rule out neurologic disease or bone lesions involving axial skeleton as the cause of lameness. AHL
Reference
Canning P. Diagnostic considerations for lameness. Ont Assoc Swine Vets Ann Mtg, King City, ON, 2016.
Summary of sample collection for diagnosis of infectious arthritis:
- joint fluid (1-2 mL, aspirated aseptically) in serum tube or other sterile container
- for Mycoplasma hyosynoviae and Mycoplasma hyorhinis PCR
- synovial swab (in transport medium, e.g., Culturette swab) – for bacterial culture
- several synovial tissue samples in formalin – for histopathology
- 1-2 fresh synovial tissue samples, in Whirl-Pak bag – to hold frozen for additional testing, if required
Parasitic pneumonia in finisher pigs
Josepha DeLay, Andrew Peregrine, Kevin Vilaca, Brent Jones
Two groups of finisher pigs developed severe, acute-onset dyspnea beginning 7-14 days following placement on straw in solid-floored barns; 70-90% of pigs in each group were affected. Parasitic pneumonia caused by larval Ascaris suum migration was suspected, based on housing conditions and on the large numbers of animals affected. The pigs were treated with injectable ivermectin (300 µg/kg subcutaneously), and most severely affected animals also received corticosteroids. Immediate and significant improvement was noted in most pigs in 1 herd, although a few (2-3) animals died. Death loss was significantly higher in the second herd, with 30% mortality, likely because of a delay in treatment. Lung and liver samples from 2 pigs in each group were submitted to the AHL for confirmation of the clinical diagnosis.
Grossly, lungs were firm and did not collapse. There was mild interlobular edema, and 1-2 cm diameter hemorrhagic foci were present multifocally in lung parenchyma. Histologically, aggregates of mixed inflammatory cells with prominent eosinophils filled alveoli and expanded interstitium, and surrounded bronchi and bronchioles. Variable numbers of nematode larvae were present in alveoli and in airway lumens, and were surrounded by hemorrhage and eosinophils. Small foci of necrosis and eosinophilic inflammation in the liver were variably associated with similar nematode larva. Streptococcus suis was isolated in large numbers from the lung of 1 pig, reflecting opportunistic infection secondary to larval nematode injury.
The severity of parasitic pneumonia in these pigs and the large number of affected animals likely reflect exposure to, and synchronous migration of, massive numbers of larvae in naive animals. Heavy environmental contamination was attributed to the flooring in the barns where these pigs were housed. Recent warm weather conditions in southwestern Ontario may have also contributed to an increase in survival of larvated eggs, resulting in a high environmental load of infective eggs.
Treatment with injectable ivermectin is necessary for rapid elimination of migrating L4 stages and resolution of clinical signs. In these herds, no additional deaths occurred after treatment, and the pigs gradually and completely recovered over 5-7 days. These cases serve as a reminder that A. suum is alive and well in Ontario, and of the importance of deworming and appropriate barn hygiene in preventing morbidity and mortalilty caused by this parasite. AHL
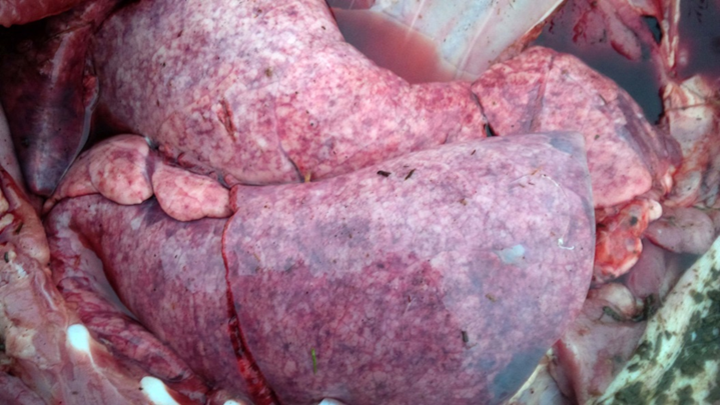
Figure 1. Lungs in situ from a pig with parasitic pneumonia caused by Ascaris suum. Note the multifocal hemorrhage and interlobular edema. (photo: Dr. K. Vilaca).
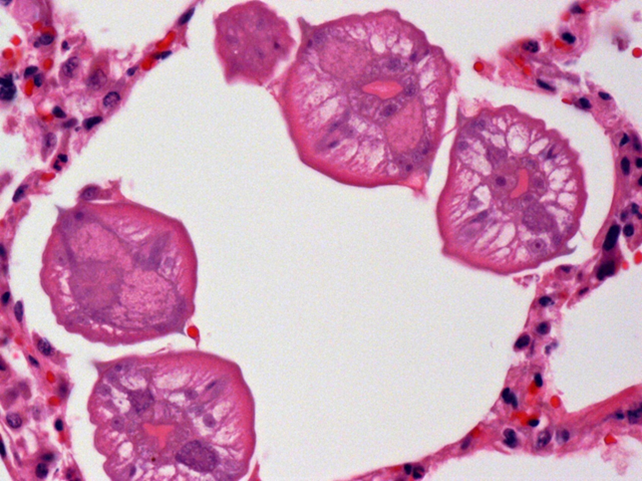
Figure 2. Several ascarid larvae in cross-section within a pulmonary alveolus.
Expanded anticoagulant rodenticide screen
Nick Schrier, Felipe Reggeti
The AHL is now offering an expanded anticoagulant rodenticide (AR) screen that covers 13 rodenticides; difethialone, flocoumafen, bromadiolone, brodifacoum, difenacoum, chlorophacinone, coumachlor (Fumarine), diphacinone, warfarin, coumafuryl, coumatetralyl, pindone, valone, and the natural occurring anticoagulant, dicoumarol. The method uses liquid chromatography–tandem mass spectrometry (LC/MS/MS) instrumentation to achieve limits of detection in the low parts per billion (ppb) range.
Suitable samples include 10 g liver, 5 mL serum, 5 mL blood (red top), and 20 g of bait/source material. Specimens should be shipped frozen, with the exception of blood, which should be refrigerated in a sealed container.
The best diagnostic samples are liver and serum. The diagnosis of AR intoxication requires both the presence of one or more AR in appropriate samples (e.g., liver or serum) and antemortem or postmortem evidence of a coagulopathy unrelated to another identifiable cause of hemorrhage (e.g., trauma). Cost of the test is $90 per sample. AHL
AVIAN/FUR/EXOTIC SPECIES
Rabbit hepatic coccidiosis- re-emerging as a clinical disease?
Marina Brash
Liver samples were submitted to the AHL for histologic evaluation from a postmortem conducted on a rabbit that was one of multiple rabbits that had died suddenly with no evidence of clinical illness. This rabbit was one of many that were housed in a large pen on a small urban farm. The only gross lesion that was identified on postmortem was a liver filled with tiny abscesses (Fig. 1) and histologically the abscesses were determined to be dilated and fibrotic bile ducts containing large numbers of coccidial organisms (Figs. 2, 3). Wet-mount examination of fluid from these cystic nodules is a useful in-clinic test that quickly demonstrates the characteristic ovoid Eimeria stiedae oocysts measuring ~30 X 20 µm (Fig. 4). Demonstration of coccidial oocysts by fecal flotation however, is not diagnostic, for the rabbits are also likely shedding intestinal coccidial oocysts that are of a mixture of species.
Enteric coccidiosis continues to be one of the important potential causes of enteritis in Ontario commercial rabbitries, but hepatic coccidiosis has seldom been identified in rabbits submitted to the AHL for diagnostic enteritis workup. However, over the last few years, there has been increased interest in the raising of small groups of rabbits for meat or as pets and these rabbits have either been raised on the ground or allowed access to the ground. Owners of these small rabbitries have contacted their veterinarians for morbidity and mortality concerns and in turn, the AHL has received increased enquiries and pathology submissions from veterinarians related to the identification of hepatic coccidiosis in these rabbits.
In commercial rabbitries, hepatic coccidiosis continues to be a concern but typically occurs as a sub-clinical disease resulting in condemnations of livers from meat-type rabbits at processing. However, if young, naive rabbits are exposed to high enough levels of sporulated oocysts, clinical disease including anorexia, poor weight gain, distended abdomens, diarrhea, and death can occur. Long-lasting immunity to Eimeria stiedae is possible, if the rabbits do not receive an excessively high exposure initially and are immunocompetent.
Coccidial oocysts are extremely resistant to environmental influences and no commonly available disinfectants will kill coccidial oocysts, so minimizing the exposure to infected feces is of great importance. This will be challenging if the rabbits are raised or are allowed access to the ground and the concern is that hepatic coccidiosis may be re-emerging as a clinical disease. AHL
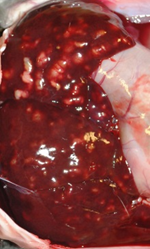
Figure 1. Characteristic appearance of hepatic coccidiosis caused by Eimeria stiedae. The liver is enlarged with numerous raised, firm, pale, variably sized, cystic, round to elongate nodules filled with turbid pale green-yellow fluid scattered throughout the hepatic parenchyma.
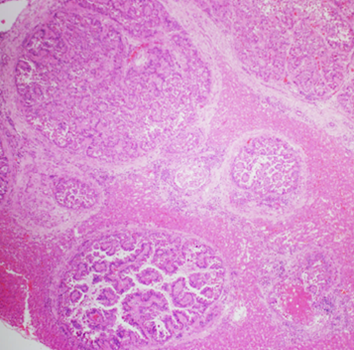
Figure 2. The liver nodules described by the veterinarian were dilated bile ducts lined by hyperplastic biliary epithelium rimmed with increased amounts of periductal fibrous tissue with compression of the surrounding hepatic parenchyma. 40X H&E.
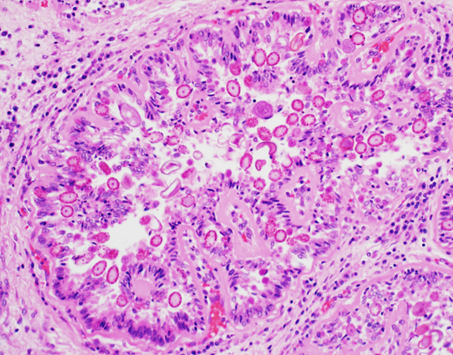
Figure 3. A dilated bile duct. Biliary epithelial cells are filled with asexual and sexual developmental stages of coccidial organisms and the lumen contains coccidial oocysts. 200X H&E.
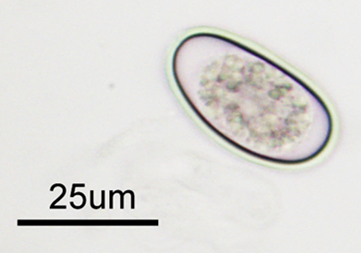
Figure 4. The characteristic ovoid Eimeria stiedae oocysts measuring ~30 X 20 µm can be demonstrated in wet mount preparations made from the fluid aspirated from the cystic liver nodules.
HORSES
Equine juvenile ossifying fibromas
Andrew Vince
Multiple deep punch biopsies were submitted to the AHL from a mandibular mass from a 5-month-old Quarter Horse; the mass had first been noted 8 wk prior and had failed to respond to antimicrobial therapy. Radiographs reportedly showed significant reactive and proliferative bone in association with the mass. Histologically, this mass was composed of slender spindle cells arranged in streams and whorls on moderate-to-large quantities of fibrous stroma. The constituent cells had poorly defined cell margins, small-to-moderate quantities of pale eosinophilic cytoplasm, oval-to-elongate nuclei, 3-fold anisokaryosis, finely granular chromatin, innocuous nucleoli, and 4 mitotic figures in ten 400x fields. Scattered throughout the mass were occasional irregular islands of osteoid containing numerous plump spindle cells typical of osteoblasts; some of these were well-differentiated, hypocellular, and mineralized; others were more hypercellular and poorly mineralized. After consultation among AHL pathologists, the consensus was that this lesion was an ossifying fibroma.
Ossifying fibromas are rare tumors of the mandible (and rarely maxilla) of young horses (Fig. 1), rarely reported in cats, dogs, and sheep, and with a similar pathologic entity in children. These masses are firm-to-hard, expansile, and arise from and distort bone. They have histologic features similar to osteomas with a larger contribution of denser fibrous stroma.
These masses are benign and slow-growing with no reported risk of metastasis. However, their location can impact normal prehension of feed and can make them difficult to resect, particularly when very large. Eight similar cases of equine ossifying fibroma were identified in a search of AHL records from 1998-2016, including 2 postmortem cases, 1 excisional biopsy, and 5 incisional biopsies. Of these, 3 were in Thoroughbreds, 2 in Standardbreds, 2 in Quarter Horses, and 1 in a Belgian horse; 5 were in males, 3 in females. The age was specified in 6 of 8 cases; the average was 6.3 years, with a minimum of 3 months and a maximum of 12 months. Seven of 8 were within the mandible, 1 in the maxilla.
Ossifying fibroma should be considered a differential diagnosis in young horses with progressively expanding masses on the mandibles or maxilla. Larger, deeper biopsies are often required for definitive diagnosis. AHL
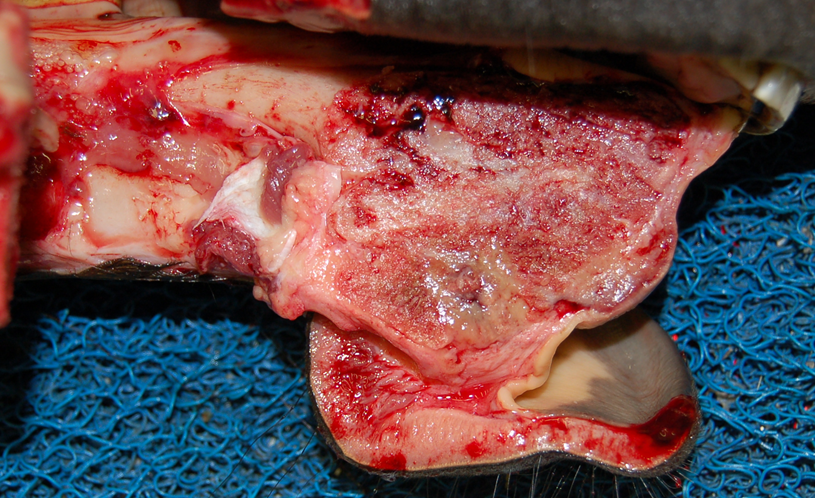
Figure 1. Juvenile ossifying fibroma in the rostral mandible of a young horse.
ACTH seasonality and PPID
Felipe Reggeti
Pituitary pars intermedia dysfunction (PPID; “Cushing’s disease”) is an endocrine condition commonly identified in aging horses and ponies. It is considered to result from loss of dopaminergic inhibition of the pituitary gland causing excessive release of ACTH into plasma and subsequent hypercortisolemia. The diagnosis may be based on clinical signs in “full-blown” cases, but it is often more difficult in animals with subtle signs or inconclusive laboratory data.
Multiple endocrinology tests have been proposed to support a diagnosis of PPID, but in 2015, the Equine Endocrinology Group (http://sites.tufts.edu/equineendogroup) recommended the use of endogenous ACTH and the TRH-stimulation test measuring endogenous ACTH (availability of TRH is currently limited in Canada). The AHL offers a chemiluminescent ACTH test, individually and within various endocrine profiles, but results must be interpreted in the context of clinical presentation and the time of the year the test was performed. The latter is of consideration because a “seasonal rise” in ACTH has been documented during the fall months, with median concentrations ~2 times the upper reference limit (although some healthy horses showed significantly higher values). This seasonal increase has an impact in the interpretation of laboratory results. Reference intervals at the AHL (2-10 pmol/L) were developed outside the seasonal rise; thus, as an example, an ACTH result of 18 pmol/L could be adequate in the fall but would be elevated the rest of the year.
The seasonal rise in ACTH may be exaggerated in early PPID, increasing the sensitivity of the test; however, elevated ACTH may be associated with other stress-related conditions and does not necessarily imply that the animal will develop PPID. Further, some of the clinical and laboratory findings of PPID overlap with other endocrine conditions, including equine metabolic syndrome (e.g., laminitis and insulin dysregulation with or without hyperglycemia), making the diagnostic interpretation challenging. AHL
COMPANION ANIMALS
A case of feline paraneoplastic alopecia
Jan Shapiro, Andrew Brooks
An 11-year-old domestic shorthair cat was submitted for postmortem examination to the Animal Health Laboratory in Kemptville. The owner had presented the cat to a veterinary clinic with the complaint that it had been ill for a couple of weeks and had hair loss. Given the very poor condition of the cat, the veterinarian recommended euthanasia.
Postmortem examination of the cat revealed dramatic alopecia, affecting at least 30% of the body (Fig. 1). The affected areas included the base of the ears, the intermandibular area, posterior and ventrolateral aspect of the mandibles and neck, pectoral area, axillae, ventrolateral thorax and abdomen, inguinal areas, anus and perineum, the underside of the tail, and large patchy areas of all 4 legs including the digits. The alopecic skin was smooth, red and often shiny, and the ventral abdominal skin was wrinkled. The hair adjacent to the alopecic areas was easily epilated.
On internal examination, the liver had numerous discrete tan-pink oval-to-stellate masses of 0.1 x 0.1 cm to 0.8 x 1.2 cm. The masses bulged from the capsular surface of the liver, and were present in the parenchyma of all lobes. The left thyroid gland was 4-fold heavier than the right, and normal thyroid tissue was compressed and displaced to one pole by an encapsulated tumor mass. The heart had moderately severe biventricular dilation, and the lungs were mottled pink and red, with mild rib impressions.
Histologic examination of skin sampled from multiple sites showed severe follicular atrophy, mild parakeratosis, mild multifocal acanthosis, and localized bacterial pyoderma. The liver tumor was diagnosed as a low-grade cholangiocarcinoma, with multinodular metastasis to the lungs. Additional diagnoses included thyroid adenoma and multifocal myocardial fibrosis.
The histopathology of the skin was consistent with that reported for feline paraneoplastic alopecia, in this case associated with malignant neoplasia of the bile ducts.
Cutaneous paraneoplastic syndromes are dermatoses associated with internal neoplasms, usually malignant. Feline paraneoplastic alopecia (FPA) is a rare dermatosis of aged cats that is characterized by symmetrical ventral body and limb alopecia, and has been associated with pancreatic or bile duct carcinoma. The pathogenesis is not known, but the alopecia is usually reported as acute in onset and progressive, occurring over as short a period as 2 wk or as long as a few months. Hair is lost from the ears, ventral chin, neck, thorax, abdomen, and legs, and the remaining hair epilates easily. As in the case reported here, areas of alopecia are often smooth, red and shiny, and pruritus is not typically reported. Alopecia may precede, be concurrent with, or follow the diagnosis of the tumor. AHL
References
Gross TL, et al. Atrophic Diseases of the Adnexa. In: Gross TL, et al. Skin Diseases of the Dog and Cat, 2nd ed. Oxford: Blackwell, 2005.
Mauldin EA, Peters-Kennedy J. Integumentary System. In: Maxie MG, ed. Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals, 6th ed. St. Louis, MO: Elsevier, 2016.
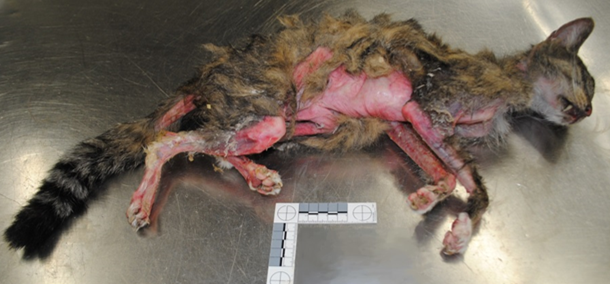
Figure 1. 11-year-old domestic shorthair cat with feline paraneoplastic alopecia associated with cholangiocarcinoma.
AHL Newsletters and LabNotes are available on the Web at - http://ahl.uoguelph.ca