AHL Newsletter, June 2017
Click here for a pdf copy of the June 2017 AHL Newsletter.
May 1, 2017, AHL User’s Guide and Fee Schedule
- Includes more test information, new tests (PCR for multiple bee viruses), new test panels, and much more!
- Also available on-line at https://www.uoguelph.ca/ahl/
- Test information is now linked to LabNotes to facilitate test selection and interpretation of results.
- We offer now a combo test for aerobic and anaerobic culture for food animals, ancultf. The anaerobic culture is recommended for abscesses and any deep wound infections.
- Please note that tumor margin evaluation, histt, is charged in addition to the histopathology fee (see page 18).
- IBRV/BoHV-1– antibody ELISA, ibre - 1 mL serum.
- New molecular and bacteriology tests for honey bees.
- New test for Myxobolus cerebralis, the myxosporean cause of whirling disease in salmonids.

Contacting the AHL
Kris Lesniewski, Jim Fairles
The AHL-Guelph to date has resisted the urge to go to a telephone tree answering system. We currently have 2 client service representatives answering phone calls at AHL-Guelph during regular business hours. (8:00 AM to 4:00 PM). AHL is open from 4:00 to 6:00 PM but with limited staffing. If all available staff are on the telephone, after several rings the call will go to voicemail. Voicemail is answered promptly once we have completed phone calls.
In order to assist us, there are several ways you can help both yourself and AHL in reducing missed calls and resulting voicemail.
- Tip: if you are looking for a particular person at the AHL that has a separate extension (veterinarians, lab supervisors), you can call 519-824-4120, and dial the extension – or: at the university switchboard, dial 0 (then you may have to dial 0 again) and you are connected to the automated system – you just need to say the name of the person you want to speak to, and you will be connected automatically (e.g., say “Jim Fairles” – and you will be connected to 54611).
- Our website – www.ahl.uoguelph.ca - for price schedule (contact ahlinfo@uoguelph.ca for user name and password), user guide, submission forms, contact information, emails and extension numbers of labs and staff. https://www.uoguelph.ca/ahl/contact-us/email-or-phone-us
- Email – non-urgent enquiries – email works best! ahlinfo@uoguelph.ca is monitored and tasks completed daily. Emails can be sent directly to the individual labs as well.
- Online access to results – if not set up already – you can get online access to results directly through our LIMS, where you can download case reports and invoices. The AHL Fee Schedule is also available here and includes external test lab locations for tests that we send out.
- Sample receipt confirmation – with this turned on, you receive a confirmation when we enter the case so you know that your submission has arrived and that the correct tests have been ordered.
Please stay tuned for more exciting things in 2017 including an improved client portal, where you enter submissions online and access and tabulate results.
Qualitative determination of desmethylbromethalin in tissue
Felipe Reggeti, Nick Schrier
Bromethalin is a non-anticoagulant neurotoxic rodenticide. It inhibits oxidative phosphorylation, limiting ATP production, which causes cell swelling, increased lipid peroxidation, and other effects. Bromethalin is rapidly absorbed from the GI tract and metabolized in the liver to desmethylbromethalin (DMB), a more potent inhibitor of mitochondrial respiration. Metabolites cross the blood-brain barrier and are very lipophilic; therefore, they readily accumulate in the brain and spinal cord causing CNS edema. The diagnosis is based upon history of exposure, clinical signs, and determination of DMB in tissues. Bromethalin was registered in 1985, but given the increasing restrictions on the use of anticoagulant rodenticides, it has become more available in recent years. Exposure of pets and non-target wildlife has been documented.
The veterinary analytical toxicology section of the Animal Health Laboratory has recently installed and verified a liquid chromatography–tandem mass spectrometry (LC-MS/MS) method developed by the California Animal Health and Food Safety Laboratory (CAHFS; Davis, California) for the qualitative determination of DMB. Fat, brain, liver and bait are suitable matrices, but because DMB is very lipid-soluble, fat and brain are the best diagnostic samples.
This is a qualitative test. A positive result indicates exposure to bromethalin, but significance needs to be interpreted in the context of clinical presentation. Cost of analysis is $90 per sample, with a 5-10 business day turnaround time.
 |
 |
|
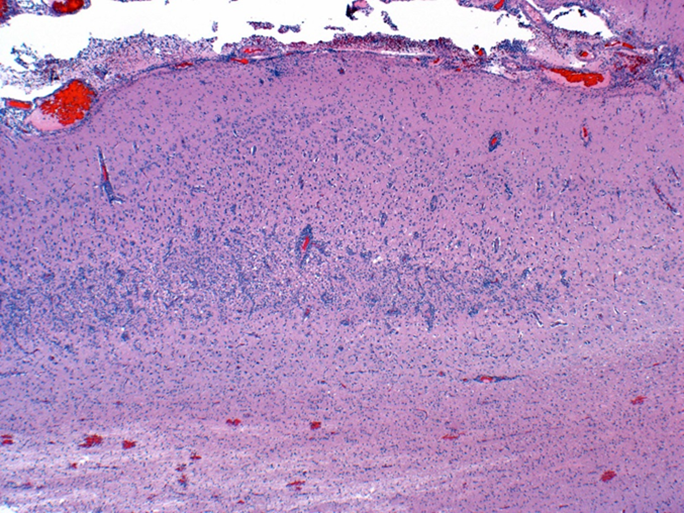
Figure 1. Subacute cerebral cortical laminar necrosis in a pig, compatible with indirect salt toxicosis. Neurons in middle cortical lamina (bracket) are necrotic, the lamina is hypercellular, and resident blood vessels are lined by hypertrophic endothelium. |
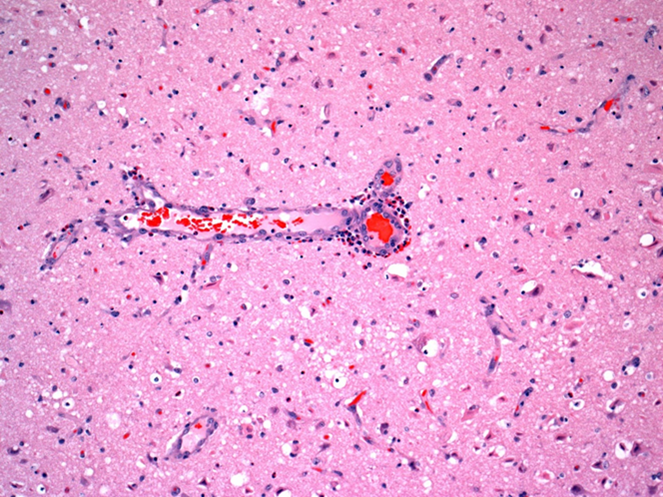
Figure 2. Indirect salt toxicosis; numerous eosinophils are in Virchow-Robin space around a blood vessel in cerebral cortex (short arrow). Many hypereosinophilic necrotic neurons are present in surrounding neuroparenchyma (long arrows). |
AHL Newsletter
June, 2017 - Volume 21, Number 2
Editor: Grant Maxie, DVM, PhD, Diplomate ACVP
Editorial Assistants: Helen Oliver, April Nejedly
The AHL Newsletter is published quarterly (March, June, September, December) by the Animal Health Laboratory, Laboratory Services Division, University of Guelph.
Its mission is to inform AHL clients and partners about AHL current activities, and laboratory-based animal disease events and disease trends. All material is copyright 2017. Ideas and opinions expressed herein do not necessarily reflect the opinions of the University or the Editor.
Articles may be reprinted with the permission of the editor and with appropriate credit given to the AHL Newsletter.
Mailing address & contact information:
Animal Health Laboratory
Laboratory Services Division, University of Guelph
Box 3612, Guelph, Ontario, Canada N1H 6R8
Phone: (519) 824-4120 ext. 54538; fax: (519) 821-8072
To receive an electronic copy of this Newsletter, please send your email address to us at holiver@uoguelph.ca
ISSN 1481-7179
Canada Post Publications number - 40064673
Contributors to this issue
- from the Animal Health Laboratory:
Melanie Barham, DVM, PMP
Michael Deane, BA
Josepha DeLay, DVM, DVSc, Diplomate ACVP
Jim Fairles, DVM, MBA
Murray Hazlett, DVM, DVSc, Diplomate ACVP
Kris Lesniewski, AHT, MLT
Beverly McEwen, DVM, PhD, Diplomate ACVP
Felipe Reggeti, DVM, PhD, Diplomate ACVP
Nick Schrier, MSc
Jan Shapiro, DVM, DipEqSurg, DipPath
Durda Slavic, DVM, PhD
Margaret Stalker, DVM, PhD, Diplomate ACVP
Andrew Vince, DVM, DVSc, Diplomate ACVP
Other contributors:
Adam Chambers, DVM; Bruce Duncan, DVM; AGCO, Toronto, ON.
Kent Charlton, DVM, Durham, ON
Ann Godkin, DVM, DVSc, OMAFRA, Guelph, ON
Our continued thanks to all of the non-author AHL clerical, technical, and professional staff who contribute to the generation of results reported in the AHL Newsletter.
OAHN Update June 2017

Rabies cases in Ontario have continued to be reported, and we have been posting weekly updated rabies maps and news on our site at OAHN.ca. Along with the ongoing rabies updates, there have been industry infectious laryngotracheitis advisories released through the Feather Board Command Centre and published in the Poultry News section of our site. The avian influenza situation in the US and abroad continues to be monitored here in Ontario, and OMAFRA has released biosecurity advisories to inform backyard flock owners and commercial producers. Follow us on Facebook and Twitter for news items every day as we collate the news for you in one place. Don’t use social media? Check out animal health links of the week, posted weekly on our website.

New podcasts! We have published new podcasts at oahn.podbean.com, including a 2-part series on Echinococcus multilocularis with Dr. Andrew Peregrine, a 2-part series on avian influenza with Dr. Tom Baker, and a 3-part series on equine proliferative enteropathy (Lawsonia) with Nathan Slovis, DVM, DACVIM, CHT (Haygard Medical Centre) and Memo Arroyo, LVM, DVSc, PhD, DACVIM.
![]()
The OAHN bee network has published a new podcast on the resources available in Ontario for beginner and experienced beekeepers. The podcast is with OBA’s Les Eccles and can be found here.
![]()
The OAHN small ruminant network met in April, with reports to be released soon. Reports for Q4 are now available on OAHN.ca and discuss Clostridium perfringens in dairy goats, iodine deficiency goiter, and more.
![]()
The OAHN bovine network is conducting focus groups– stay tuned! There is a new national surveillance group called CAHSS-Bovine - see www.cahss.ca for more.
![]()
The OAHN Fish Network released its 4th quarter report this spring, which includes a disease summary, provincial update, federal update, and an update on the OAHN Research project. Report is available here.
![]()
The OAHN swine network met in April, and its report was released in May. Network reports can be found here. Recently, the network has focused on erysipelas and PED. The network’s reports continue to do well in their distribution through OASV and Ontario Pork.
![]()
On OAHN alternative species calls, we discuss interesting cases with experts, and we have a listserv to trade case and treatment ideas. Email oahn@uoguelph.ca to join.
![]()
The OAHN poultry network held its last call in March, and its latest survey was released on Feb. 1. The network has also held calls in response to new strains of IBV. You can check out the IBV Fact Sheet here.
The network worked with OMAFRA to map IBV positives in 2016 to the county level. Look for information in the next OAHN report.
Are you a small flock veterinarian? Join our small flock vet listserv by emailing oahn@uoguelph.ca
![]()
The OAHN equine network’s latest reports can be found here. In these reports, the network discusses: seroprevalence of Borrelia burgdorferi and Anaplasma phagocytophilum infection in Ontario horses, vitamin E testing, and supplementation, skin disease, and more.
![]()
The CWHC Q1 2017 report was published in April. Find the report here. Through its OAHN Project, the wildlife network has launched a citizen surveillance website to report wildlife disease. Find it here: http://wildlifehealthtracker.com
![]()
The OAHN companion animal network released its quarterly report in April, covering Lyme disease, respiratory antimicrobial resistance guidelines, and rabies. Keep your eye on our podcast page for an upcoming recording covering raw diets. As well, OAHN published part 2 of a podcast series on E. multilocularis, featuring interviews with Dr. Andrew Peregrine and master’s student Jonathon Kotwa.
RUMINANTS
Nutritional chondrodystrophy in beef calves
Josepha DeLay, Kent Charlton, Ann Godkin, Felipe Reggeti
A 200-cow Simmental herd in SW Ontario experienced a cluster of births in which calves had prominent congenital defects. Of the 12 calves born during the early calving season, 10 had noticeably shortened legs and either died immediately (2 calves), or within 12 h after birth (1 calf), or survived but could not stand (7 calves). Herd genetics were stable and were similar to those of several other herds, none of which had identified similar congenital lesions.
Two of the calves that died shortly after delivery were submitted to the AHL for postmortem. Both calves were fully haired and crown-rump length was compatible with full-term gestation. All limbs were short compared to body length (disproportionate dwarfism, Fig.1). There was no evidence of arthrogryposis or spinal deviation. Thyroid gland was markedly enlarged in one fetus, and mildly enlarged in the second fetus, consistent with goiter. BVDV nucleic acid was not detected in thoracic fluid of either fetus by PCR.
Histologically, lesions were limited to thyroid glands and physes of long bones, including distal femur, proximal tibia, distal humerus, and proximal and distal radius. Physes were irregularly thin, and there was evidence of chondrodysplasia and premature closure, with a thin discontinuous band of bone oriented parallel to the epiphyseal aspect of the physes. Cartilage was poorly organized, most prominently in the zone of chondrocyte hypertrophy. Lesions in thyroid gland confirmed colloid goiter. Liver manganese levels in both calves were in the deficient range (0.72 and 0.79 µg/g, RI 2-6 µg/g).
Lesions involving physes of long bones in both calves were responsible for the decreased length of these bones and resulting short stature of the calves. In combination with low liver manganese levels, these results support a diagnosis of congenital nutritional chondrodystrophy associated with maternal micronutrient (manganese) deficiency during gestation. Concurrent iodine deficiency is suggested by the presence of goiter in both calves.
Disproportionate dwarfism has previously been associated with manganese deficiency in calves in Canada, New Zealand, and elsewhere. Joint laxity and swelling is also described in affected animals. Low manganese levels in feed have been linked to drought conditions during the previous growing season, as well as to diets inherently low in manganese, including unsupplemented corn silage, as was fed in this herd. Manganese contributes to pathways of glycosaminoglycan synthesis, and deficiency of this essential micronutrient disrupts normal endochondral ossification and bone development. Zinc deficiency is suspected to contribute to similar congenital lesions, although adequate tissue zinc levels were identified in these calves.
 |
 |
 |
 |
|
Figure 1. Disproportionate dwarfism in a neonatal Simmental calf. |
Figure 2. Distal femoral physis – normal (control) calf. Cartilage has an orderly arrangement and maturation sequence, with chondrocytes oriented in discrete parallel columns. |
Figure 3. Distal femoral (A) and humeral (B) physes in calf with congenital nutritional chondrodystrophy. Physeal cartilage has a disorganized appearance, with lack of orderly columnar arrangement of chondrocytes and distinct zones of hypertrophy and maturation. |
|
Abortion caused by Candida spp. yeast in a Holstein cow and a Warmblood mare
Margaret Stalker, Beverly McEwen, Durda Slavic
A 30-cm crown-rump length, 4-mo Holstein aborted fetus and placenta were received at the AHL for postmortem examination in late February 2017, from a herd with a history of multiple abortions 4-6 mo into pregnancy. On histology, there was locally extensive necrotizing placentitis with yeast organisms visible within placental trophoblasts. The fetus had pneumonia with scattered multinucleate cells in airway lumens, as well as conjunctivitis, and colitis with numerous yeast organisms visible within the lumen of the colon. The yeast organism grown in large numbers from the placenta and abomasal content was identified as Candida parapsilosis by MALDI-TOF mass spectrometry.
In early March 2017, an aborted 7-mo gestation Warmblood equine fetus and placenta were received for postmortem. Histologic examination of the chorion revealed necrosis and yeast organisms within trophoblasts, particularly evident in sections from the cervical star area, and the amnion. There was fetal pneumonia with large numbers of budding yeast organisms in alveoli, with rare multinucleate cells. Large numbers of Candida tropicalis were identified in placenta, fetal lung and stomach content fluid.
Candida spp. are dimorphic fungi with both a yeast and a mycelial phase. Although typically considered commensal inhabitants of the digestive and genital tract, these fungi are opportunistic pathogens. Alterations in host mucosal environment or defense mechanisms can result in overgrowth and systemic invasion. Placental localization is assumed to be associated with hematogenous spread following a breach in normal mucosal barriers, although contamination of the uterus during artificial insemination has also been suggested in some cases.
Candida spp. have been implicated as an uncommon cause of sporadic infertility and reproductive problems in cattle, including abortion. A search of the AHL database (2007 to 2017) revealed 6 cases of abortion, all in Holsteins, associated with yeast infections, including C. parapsilosis (2 cases), C. krusei (1 case), Candida spp. (2 cases) and Rhodotorula sp. (1 case).
Candida spp. have also been recognized as causing endometritis and early embryonic death and infertility in mares; Candida abortion in mares is considered to be very rare. There is a single case report of abortion caused by Candida parapsilopsis, and a single case report of C. guilliermondii. Presumably this was an opportunistic ascending infection, and the same risk factors for fungal endometritis (poor uterine contractility, anatomical malformations, and inefficient uterine defense mechanisms) may play a role in allowing establishment of infection.
AVIAN/FUR/EXOTIC SPECIES
Further demise of the penny
Margaret Stalker
An adult pied imperial pigeon was submitted to the AHL for postmortem examination. The bird was found dead, with green mucus draining from the oral cavity. Postmortem examination revealed the bird to be in thin body condition. Internally, the most striking finding was an enlarged and dilated gizzard lined by thickened, soft and friable koilin with multiple erosions, containing bright green mucus and a single, extensively eroded 1999 Canadian penny (Fig. 1). In addition to ventriculitis, histology revealed enteritis, as well as hemosiderophages in the liver, extensive tubular degenerative changes in the kidneys, and to a lesser extent, the pancreas. Trace mineral analysis of the pancreas revealed zinc levels of 2,400 µg/g, well into the toxic range (1,000-3,500 µg/g), confirming the diagnosis of zinc toxicosis.
Although dietary zinc intake is required for various physiologic processes including the function of numerous metalloenzymes, excessive intake of the heavy metal can have detrimental effects. Zinc toxicosis is well-documented in mammalian and bird species, particularly in inquisitive psittacines chewing on the surfaces of galvanized metal cages (“new wire disease”) or ingesting cage accessories, hardware, and metallic toys. Clinical signs are often nonspecific and include depression, lethargy, anorexia, weight loss, regurgitation, anemia, PU/PD, ataxia, paresis, anemia, and sudden death. The pancreas appears to be a major target organ of Zn toxicity, although other target organs include the kidney, gastrointestinal tract, and liver.
This large pigeon was able to ingest a coin, and it certainly chose the wrong one! Prior to 1997, Canadian pennies were composed of 98% copper with traces of tin and zinc. From 1997-1999, pennies were composed of 98% zinc with a copper plating, and from 2000 onwards of 94% steel, with a copper plating. Unfortunately for this bird, the minting date sealed its fate.
 |
|
Figure 1. Eroded 1999 penny in the ventriculus of a pied imperial pigeon. |
A case of duck viral enteritis
Jan Shapiro
In April 2017, a mature Muscovy duck hen from a hobby flock was submitted for postmortem to the AHL-Kemptville lab under the Small Flock Disease Surveillance project. The duck was from a small farm with 19 ducks of various breeds, 3 turkeys, 30 laying hens, and 5 quail. Each bird species was kept separately, both in outside pens in the day and in separate sheds at night. The hen had been eating and seemed healthy, but was found dead. The day after the hen was submitted, the 2 other Muscovy ducks died but were not submitted.
At postmortem, the hen was in good body condition, with generalized tissue congestion and petechiation of abdominal fat and epicardium. There was multifocal to confluent severe acute necrosis and ulceration of the mucosa of the esophagus. The small intestinal, large intestinal, and cecal content was dark fluid, and the intestinal lymphoid tissue (annular bands) was transmurally congested and dark red.
Histology revealed severe acute necrotizing esophagitis, enteritis characterized by villus atrophy, acute crypt necrosis and multifocal secondary bacterial infection, severe acute lymphoid necrosis of intestinal annular bands (Fig. 1)and spleen, mild multifocal hepatic and bile duct necrosis. Eosinophilic intranuclear inclusion bodies were seen in esophageal squamous and glandular epithelial cells, gut crypt epithelium, reticular cells in lymphoid tissue, bile duct epithelial cells, and hepatocytes. PCR was negative for Newcastle disease (APMV-1) and influenza A viruses.
The gross lesions and histopathology are typical of duck viral enteritis (DVE, duck plague), caused by species Duck herpesvirus 1 (DHV-1). The PCR test for DHV-1, conducted by Texas A&M Veterinary Medical Diagnostic Laboratory, was positive (esophagus, lung, spleen). Anecdotally, DVE is rarely diagnosed in Ontario in wild or captive ducks.
Domestic ducks can be infected by direct contact with wild birds or contact with the contaminated environment, commonly water. Recovered birds may carry the virus in its latent form, and viral reactivation may be the cause of outbreaks. Wild birds can be asymptomatic carriers, shedding virus intermittently for years. Some birds have a short period of nonspecific illness followed by death. Breeder ducks can have sudden high mortality. As infection moves through the flock, more signs are seen, including a severe drop in production, anorexia, thirst, depression, weakness, nasal and ocular discharges, diarrhea, and sometimes neurologic signs. Young ducklings can have severe diarrhea with some blood, and die rapidly. Morbidity and mortality can be very high, and Muscovy ducks seem to be more susceptible than other duck breeds.
 |
|
Figure 1. Small intestine annular bands showing mucosal hemorrhage and lymphoid necrosis. H&E. |
SWINE
Indirect salt toxicosis in swine
Josepha DeLay, Murray Hazlett, Margaret Stalker, Andrew Vince
Samples from nursery and finisher pigs from 3 farms with suspected salt toxicosis were submitted to the AHL in April and May 2017. In herd 1, seizures and apparent blindness were noted in 50% of pigs following a period of water deprivation, and indirect salt toxicosis was suspected clinically. Sudden-onset neurologic signs in herd 2 included seizures, head pressing, and star-gazing. High mortality was noted in herd 3, without mention of specific neurologic signs. Salt toxicosis was listed as a clinical differential diagnosis in herds 2 and 3, although water deprivation was not noted.
Grossly in cerebral cortex of some pigs, there was distinct separation along necrotic laminae. Variably severe laminar cortical necrosis was also evident microscopically (Fig. 1, p. 14). Blood vessels in meninges and cerebral cortex were surrounded by variable numbers of eosinophils mixed with fewer lymphocytes and plasma cells (Fig. 2, p. 14).
Pathologic findings of eosinophilic meningoencephalitis and concurrent laminar cerebral cortical necrosis are virtually pathognomonic for “salt toxicosis” in pigs. The clinical syndrome in pigs is actually one of “indirect salt poisoning” which is a more accurate name for the condition. This is most often due to inadequate water availability and intake, sometimes but not necessarily with a concurrent increase in dietary salt levels. The condition has also been linked to whey feeding. High serum, tissue, and CSF sodium levels precipitate cerebral anoxia, with sudden access to water likely causing an osmotically induced cerebral edema and necrosis.
Since 2007, 10 cases of salt toxicosis have been diagnosed by pathologists at the AHL. The occurrence of the 3 recent cases over a < 2 mo time span is unusual.
HORSES
Actinobacillus equuli ssp. equuli valvular endocarditis in a horse
Murray Hazlett, Adam Chambers, Bruce Duncan, Durda Slavic
A 3-year-old male Standardbred horse with a history of recurrent illness including colic with weight loss was euthanized. At autopsy there was an organized 15-cm thrombus in the right jugular vein (Fig. 1A) that histologically was septic, containing neutrophils and bacteria. Large numbers of needle punctures were present in the left jugular. An irregular 2 x 3 cm vegetative proliferation was found on the right atrioventricular valve (Fig. 1B), and microscopically there was fibrosis and inflammation within the right AV valve. The large vegetative mass on the valve was composed of fibrin with enmeshed neutrophils and large colonies of bacteria.
Hemorrhagic striae were present within the serosa of the small intestine (Fig. 1C). There was typhlocolitis (Fig. 1D) with severe mucosal edema and ulceration from the cecum to the right ventral colon, with formed feces in the descending colon. Bone marrow had hyperplasia of the neutrophil series, likely the result of chronic inflammation. Multifocal embolic pneumonia was seen histologically.
The right AV valve was cultured, and large numbers of A. equuli subsp. equuli were isolated. Testing of the colon for Potomac horse fever, Clostridium difficile, Salmonella spp., and coronavirus was negative. There was a suspicious PCR reaction for ruminant rotavirus B.
A. equuli subsp. equuli is commonly found in the equine oropharynx and intestinal tract, and is associated with septicemia in foals and sometimes adult horses. In adult horses, it has been associated with cellulitis and peritonitis.
There have been several reports of A. equuli valvular endocarditis in horses as well as in septicemia in humans and pigs. In this case, because of the septic thrombus “upstream”, it was felt that the A. equuli colonized the heart valve from repeated hematogenous showering. The embolic pneumonia may have been from the jugular thrombus, the heart valve, or both. Contamination of the thrombus may have been from oral fluid or feces from the horse.
It is uncertain if the typhlocolitis was associated with dysbacteriosis associated with treatment with antibiotics, or if the repeated jugular injections were an effort to treat the colitis. The colitis was of some duration, as there was submucosal fibroblast activation and some fibroplasia in response to the ulceration and edema.
 |
|
Figure 1. Actinobacillus equuli ssp. equuli valvular endocarditis in a horse. A. Organized thrombus in the right jugular vein (arrows). B. Vegetative mass on the right AV valve of the heart (arrow). C. Unusual hemorrhagic striae on the small intestinal serosa. D. Colonic ulceration and edema (arrows). |
COMPANION
Companion animal histopathology tests at the AHL
AHL histopathology tests and associated fees for companion animal biopsies and in-clinic postmortems are categorized based on the number and size of samples. Please be sure to read the details for each subset of test so that you select the correct test for each case.
Also please note that for surgical margin evaluation of biopsies >2 cm diameter, the ‘tumor margin evaluation’ test must be selected in addition to the appropriate histopathology test, and an additional fee will apply for the evaluation on these large tumors.
|
Test name |
Test code |
Details |
|
Histopathology, 1-2 biopsies or tissues |
histcm1 |
For submissions with 1-2 biopsies or tissues, OR multiple (6 or fewer) punch, Tru-Cut, or endoscopic biopsies |
|
Histopathology, 3-6 biopsies or tissues |
histcm2 |
For submissions with 3-6 biopsies or tissues, OR 6-10 cm diameter |
|
Histopathology, 7 or more biopsies or tissues |
histcm3 |
For submissions with 7 or more biopsies or tissues, OR >10 cm diameter (e.g., large tumors, spleen, brain, mammary chain, heart) |
|
Tumor margin evaluation |
histt |
Applies in addition to regular histopathology charge. For tumor excisional biopsies >2 cm diameter. Includes preparation of 4 radial margin sections. Must be requested at time of sample submission. |
Systematic approach in diagnostic toxicology
Felipe Reggeti
Veterinarians frequently contact the AHL inquiring about toxicology testing for suspect intoxications or malicious poisoning, but the available information is often times limited. A toxicologic investigation is commonly far from straightforward, and requesting toxicology tests without clear direction can be unrewarding, expensive, and frustrating. Presumptive exposure does not necessarily equal intoxication.
- A systematic approach needs to be followed, beginning with a detailed clinical history to identify potential sources, including changes in management, recent applications of pesticides, storage of chemicals and drugs, etc.
- The veterinarian needs to perform a thorough physical exam and collect clinicopathologic data from CBC, clinical biochemistry, and urinalysis to narrow down the list of differential diagnoses. Underlying infectious, metabolic and other conditions that may have similar presentations need to be ruled out before continuing with toxicology testing. Clinical biochemistry may provide evidence that specific systems have been targeted (e.g., hepatic and renal).
- If the case involves deaths, a complete postmortem examination is essential, including sampling of tissues for microbiology, histopathology, and toxicology. Some samples may not be necessary but should be available if needed (keep toxicology samples frozen). A postmortem exam is probably the most important aspect of the investigation, as it may indicate that toxicology testing is unnecessary. Alternatively, it could reveal lesions that are relatively specific for some toxins, or no obvious lesions at all (e.g., organophosphates).
The Toxicology laboratory at the AHL offers a wide array of tests for chemical analysis that may be helpful to support or confirm the presumptive intoxication. A few bioassays are also available; e.g., mouse inoculation test for botulism (bacteriology lab). Significance of any toxicology results must be interpreted in the context of clinical presentation and other laboratory findings.
Recommended reading: Osweiler GD. Diagnostic guidelines for ruminant toxicoses. Vet Clin North Am Food Anim Pract 2011;27:247-254.
For more information on toxicology and available tests, please see the AHL User’s Guide (https://www.uoguelph.ca/ahl/tests-users-guide/ahl-users-guide).