AHL Newsletter September 2017
Click here for a pdf copy of the September 2017 AHL newsletter.
In memoriam: Brent Hoff, DVM, DipSAMed, DVSc, DipTox

Many of our readers will have heard of the death, on June 29, 2017, of Dr. Brent Hoff, retired AHL clinical pathologist/toxicologist.
Brent was an OVC 1969 DVM graduate who went on to earn several more degrees, including a DVSc and a Diploma in Toxicology. As a practitioner, he was the owner of the Taunton Road Animal Hospital in Oshawa. He became the OMAFRA Vet Lab Services clinical pathologist in 1983 after gaining his DVSc in clinical pathology, and latterly was the AHL clinical pathologist and toxicologist until his retirement in 2013. In addition to being well-known to veterinarians in Ontario, he was an enthusiastic participant in the veterinary toxicology community in North America.
Brent had multinational veterinary experience in Canada, Norway, New Zealand, and the Turks and Caicos, and provided terrific client service in all of these pursuits. He was also an accomplished woodworker, and an instrument-rated commercial pilot.
Since retirement, Brent worked part-time for the AHL as toxicologist and mentor to Dr. Felipe Reggeti, our current clinical pathologist/toxicologist. Brent loved toxicology and was looking forward to more contract work with us on his return from New Zealand this spring, but his plans were cut short by cancer.
A celebration of Brent’s life was held in Guelph on August 20, and included a wide spectrum of family, veterinary colleagues, and other friends. Brent had a very full and fulfilling life, and enriched the lives of others. He will be missed! AHL
Disease surveillance in non-commercial poultry flocks: project ends Friday September 29, 2017
Since October 1, 2015, the University of Guelph and OMAFRA, through the Animal Health Laboratory (AHL) and the Ontario Animal Health Network, have been conducting a baseline surveillance study to determine the most common infectious diseases found in small flocks in Ontario. AHL-Guelph and AHL-Kemptville have been accepting submissions of sick/dead birds for postmortem analysis and further testing.
This 2-year project will end as of Friday September 29, 2017. After this date, all small flock submissions to the AHL will be charged based on the published AHL Fee Schedule. AHL
Update on anthrax testing at the AHL
Hugh Cai, Maria Spinato, Patricia Bell-Rogers
The AHL has validated and implemented a real-time PCR for the detection of Bacillus anthracis from clinical samples. The published assays (Ciesik et al., 2015) detect B. anthracis by targeting the chromosomal marker and a lethal factor. The assays were found to be sensitive and specific at the AHL. A commercial kit using similar principles was also validated at the lab. AHL pathologists will perform a rapid screening antigen ELISA on suspect samples to rule out anthrax. The sample will then be forwarded to the Molecular Biology Lab for PCR confirmation. AHL
Urinalysis quiz - see Companion Animals page for more information
A.
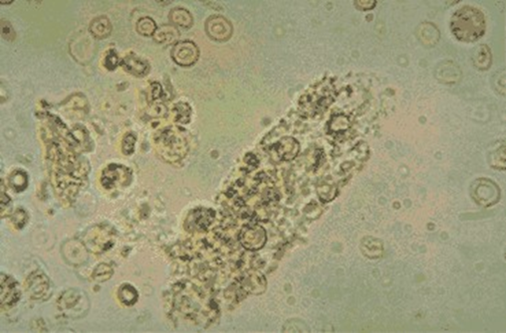
B.
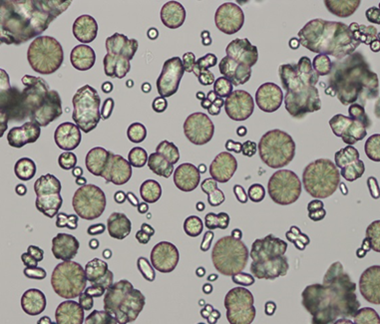
C.

A = granular cast, dog
B = calcium carbonate, horse
C = struvite, dog
Update on discontinuing Salmonella phagetyping
Durda Slavic
Among many Salmonella spp. isolated at AHL and sent to the OIE Salmonella reference laboratory for serotyping, only Salmonella Enteritidis, Salmonella Heidelberg ,and Salmonella Typhimurium and its variants underwent phagetyping. As of August 31, 2017 phagetyping of non-human Salmonella spp. isolates will be discontinued. The phage stocks required for phagetyping were provided by Public Health England, which has changed their approach to serotyping and phagetyping by moving to whole genome sequencing (WGS) of Salmonella isolates, significantly reducing phage production and making them unavailable for routine typing of non-human isolates. AHL
AHL Newsletter
September, 2017 - Volume 21, Number 3
Editor: Grant Maxie, DVM, PhD, Diplomate ACVP
Editorial Assistants: Helen Oliver, April Nejedly
The AHL Newsletter is published quarterly (March, June, September, December) by the Animal Health Laboratory, Laboratory Services Division, University of Guelph.
Its mission is to inform AHL clients and partners about AHL current activities, and laboratory-based animal disease events and disease trends. All material is copyright 2017. Ideas and opinions expressed herein do not necessarily reflect the opinions of the University or the Editor.
Articles may be reprinted with the permission of the editor and with appropriate credit given to the AHL Newsletter.
Mailing address & contact information:
Animal Health Laboratory
Laboratory Services Division, University of Guelph
Box 3612, Guelph, Ontario, Canada N1H 6R8
Phone: (519) 824-4120 ext. 54538; fax: (519) 821-8072
To receive an electronic copy of this Newsletter, please send your email address to us at holiver@uoguelph.ca
ISSN 1481-7179
Canada Post Publications number - 40064673
Contributors to this issue
- from the Animal Health Laboratory:
Melanie Barham, DVM, PMP
Patricia Bell-Rogers, BSc, MSc
Marina Brash, DVM, DVSc, Diplomate ACVP
Hugh Cai, DVM, MSC, DVSc
Michael Deane, BA
Jason Eidt, BSc
Emily Martin, DVM, MSc
Kristiina Ruotsalo, DVM, DVSc, Diplomate ACVP
Jan Shapiro, DVM, DipEqSurg, DipPath
Durda Slavic, DVM, PhD
Maria Spinato, DVM, DVSc, Diplomate ACVP
Margaret Stalker, DVM, PhD, Diplomate ACVP
Qiumei You, DVM
Other contributors:
Jeff Caswell, DVM, DVSc, PhD; Andrew Peregrine, BVMS, PhD, DVM, DipEVPC, DipACVM, Pathobiology, OVC, Guelph, ON.
Hanadie Nur, BVM&S, Toronto, ON
Our continued thanks to all of the non-author AHL clerical, technical, and professional staff who contribute to the generation of results reported in the AHL Newsletter.
Ontario Animal Health Network
 “Your comprehensive source for animal health information."
“Your comprehensive source for animal health information."
OAHN Update, September 2017
This past summer, we were focused on spreading the word about vector-borne diseases through our social media accounts. We regularly posted educational and news articles about ticks, Lyme disease, and mosquito-borne diseases that can affect people in Ontario. In addition, we put together a Vector-Borne Disease resource page, which includes a lot of these links. Check it out, and let us know if you have more resources to add. In addition to our vector-borne focus, we have been publishing Ontario rabies map updates every week. Follow us on Facebook and Twitter for news items every day as we collate the news for you in one place. We also have a page compiling alerts on the Porcine Epidemic Diarrhea (PEDV) in Western Canada (you must be logged in to view).
Don’t use social media? Check out animal health links of the week in the OAHN News Section.
 Focus on raw food
Focus on raw food
We have published a new podcast series on Raw Food Diets, featuring Dr. Scott Weese and Dr. Adronie Verbrugghe from the OVC —we approach the subject from an infectious disease perspective, and a nutritionist perspective. You can access the podcast series here. We also have completed our Bee Series, where we discuss bee resources in Ontario, lab resources, Niagara College’s beekeeping program, and more. Click here to listen.
To check out all of our podcasts, go to OAHN.podbean.com. In addition to the podcasts, we have put together a Raw food resources page, which includes links to CDC information, disease fact sheets from PHAC, and much more.
Benefits of registering on OAHN.ca
If you’re a veterinarian in North America, or if you’re an RVT in Ontario, you are eligible to register for OAHN. ca.
Registering for OAHN.ca gives you access to:
· Veterinary reports and resources
· Clinical impression summaries from quarterly veterinary surveys
· Quarterly lab data summaries
· Condemnation data
· Veterinary courses (such as our small flock veterinary course)
· Disease updates meant only for veterinary professionals (e.g., CSHIN PED Outbreak Updates)
Disease fact sheets
OAHN regularly publishes disease fact sheets and infographics throughout the year, for all species and diseases that affect animals in Ontario. Recently, we published a fact sheet entitled: Bats, Birds, Insects and Snails - A Recipe for Potomac Horse Fever. Check out this fact sheet for information on what Potomac horse fever is, how it’s spread, how it’s diagnosed, treatment, and vaccines.
The Fish Network created two infographics about whirling disease, for fish farmers and recreational water users. If you haven’t heard of whirling disease, it is a fatal, infectious disease that affects freshwater finfish. Overall death of infected fry and fingerlings can reach 90%. Susceptible species include salmon, trout, and char, all found in Ontario. It has not been found in Ontario, but cases have been recorded this year in Alberta.

*New blog posts*
Each month we will be posting new blog-style articles on OAHN.ca. So far we have discussed how we find our news articles, and how veterinarians are involved with OAHN. We will be posting new articles about National Disease Surveillance, and how producers interact with OAHN.
Do you ask your clients for their Premises IDs? Check out how fast and easy it is to do by registering your vet clinic: https://www.ontarioppr.com/home_en.html
RUMINANTS
Outbreak of parasitic pneumonia in a herd of beef cattle
Jan Shapiro, Andrew Peregrine, Jeff Caswell
In late June 2017, 2 mature beef cows from a herd of 61 cows and calves in eastern Ontario were submitted for postmortem to the AHL-Kemptville. Five cows had died in 11 days, and many others had a raspy cough. The calves were not affected. The herd was not vaccinated or dewormed. The cattle were kept on pasture, with access to a run-in area in a barn.
Cow A had been ill for 2 days, but was not treated and died. Cow B was the index case in the herd, and had been sick for 11 days. She was not treated and seemed to be improving gradually, but died unexpectedly. At postmortem, cow A was in very thin body condition, and cow B was emaciated. Significant gross lesions were confined to the lungs. The cranial and middle lung lobes of both cows and the cranioventral 1/2 of the right and left caudal lobes of cow B were mottled red and dark-red, and had a rubbery-to-firm texture. It was estimated that 55% of the lung mass of cow B was affected. Interlobular septa were mildly distended with edema or emphysema. No exudate could be expressed from airways. Bronchial lymph nodes were markedly diffusely hyperplastic.
The postmortem diagnosis was acute interstitial pneumonia. PCR testing for bovine respiratory syncytial virus, parainfluenza 3 virus, and bovine herpesvirus 1 was negative.
Histopathology of the lungs of both cows was dramatic. The lumen of alveoli and bronchioles was filled with eosinophils, non-degenerated neutrophils, macrophages, and multinucleate giant cells. Alveolar hyaline membranes and fibrin balls were prominent, and there was multifocal acute alveolar hemorrhage. Alveolar type 2 hyperplasia was marked and there were scattered syncytial cells. Interalveolar septa were expanded by lymphocytes and eosinophils; septal fibrosis was mild to severe. Eosinophils were in bronchiolar lamina propria, and transmigrating bronchiolar epithelium. In one cow, occasional bronchioles had focal attenuation or hyperplasia of lining epithelium, some were ruptured, and some had peribronchial fibrosis. Edema and a diffuse infiltrate of eosinophils admixed with smaller numbers of neutrophils and macrophages expanded interlobular septa and pleural lymphatics. There was multifocal parenchymal caseous necrosis and eosinophilic granulomas, which were occasionally mineralized. Necrotic and inflammatory foci were often centered on intact or degenerating nematode parasites. Parasites had an eosinophilic cuticle, with 2 spines (alae) and a large intestinal tract (Fig. 1), and were interpreted as ascarid larvae. The histologic diagnosis was severe parasitic bronchointerstitial pneumonia.
Dictyocaulus viviparus, the pathogenic bovine lungworm, is a trichostrongyle. However, it is unusual to see this parasite cause disease in Ontario in June and in cattle that have been on pasture for more than one grazing season. Furthermore, the morphology of nematodes in the lungs of these cattle was more consistent with ascarids. Additional history subsequently provided by the owner was that in one corner of the run-in for the cattle, there was a manure pile that had been used for the past 2 years to store manure from the pen of a small group of pigs that were kept in an adjacent area of the barn. The cattle had access to this manure pile.
The history of access to pig manure, and the histologic lesions are consistent with a presumptive diagnosis of aberrant Ascaris suum migration, but unambiguous identification of the parasites would require gene sequencing of the parasite.
After receiving the postmortem report, the owner dewormed the cattle, restricted access to the pig manure pile, and reported that there have been no new cases of respiratory disease. AHL
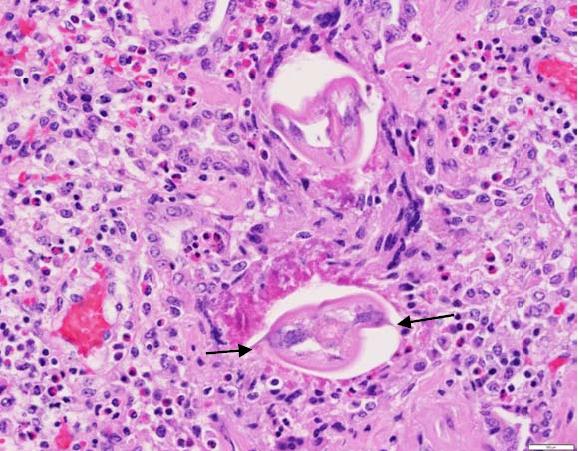
Figure 1. Lung section showing eosinophilic inflammation and cross section of parasites. Note lateral alae (arrows).
AVIAN/FUR/EXOTIC SPECIES
Clinical toxoplasmosis in a pet rabbit
Marina Brash, Hanadie Nur, Margaret Stalker
A pet rabbit was presented to a veterinary emergency clinic with a clinical history of inappetence. The rabbit was dehydrated, and radiographs showed an enlarged liver. The rabbit was referred to The Links Road Animal & Bird Clinic for further evaluation. The following day, the rabbit became pyrexic (41.7oC), and over the course of the next 24 h declined rapidly, becoming dull, depressed, exhibiting head pressing and a hunched posture indicative of abdominal pain. The owner elected for euthanasia.
At postmortem, the liver was enlarged and mottled red/tan. The spleen was enlarged and congested. A small amount of clear fluid was present in the thoracic cavity and the heart was pale. Significant histologic lesions were limited to the spleen, liver, and lung. There was acute multifocal splenic necrosis with fibrin exudation, and in close proximity to a few of these foci were clusters of small basophilic organisms (Fig 1), confirmed to be Toxoplasma gondii cysts and tachyzoites by immunohistochemical staining (Fig 2). There was also acute multifocal hepatic necrosis and interstitial pneumonia.
T. gondii is an obligate intracellular coccidium that infects many species of warm-blooded animals, including people, however clinical infections in domestic rabbits are not common. Pyrexia and lethargy have previously been reported in a rabbit dying with toxoplasmosis. The affected rabbit in the report was housed with other rabbits in hutches. Other pets in the household included several cats and dogs, and these pets had access to the area where the rabbits were housed, and where the bags of rabbit pellets were stored, affording the opportunity for food and water contamination with cat feces.
Toxoplasmosis should be included in the list of conditions that have short clinical courses culminating in mortality in rabbits, especially if they have known contact with cats. AHL

Figure 1. A focus of acute fibrinous splenic necrosis with a few variably sized clusters (arrows) of Toxoplasma gondii tachyzoites at the outer margin. 600X H&E

Figure 2. Spleen. Positive immunostaining of Toxoplasma gondii cysts and tachyzoites in the spleen. 600X
Developing a health and disease surveillance network for Ontario mink farms: project ends Friday September 29, 2017
Emily Martin
Funding was obtained through the Ontario Animal Health Network (OAHN) to provide semi-annual testing on found-dead animals for the spring and fall of 2016 and 2017. There was also funding for Aleutian disease CIE testing in the fall.
This project will be wrapping up with an end date of Friday, September 29, 2017. After this date, all mink submissions to the AHL will be charged based on the published AHL Fee Schedule. AHL
SWINE
Molecular typing of Mycoplasma hyopneumoniae field strains using p146 gene sequencing
Qiumei You, Jason Eidt, Hugh Y. Cai
Mycoplasma hyopneumoniae is the causative agent of zoonotic pneumonia world-wide. Genetic strain typing of the pathogen can be useful for disease diagnosis and management. Current M. hyopneumoniae molecular typing methods include multilocus variable number tandem repeat analysis (MLVA) assay and multilocus sequence typing (MLST). Although these molecular methods can generate useful information, they are complicated to run, somewhat difficult to interpret, and not affordable for routine testing. The serine repeat encoding region of the p146 gene was found to be highly pleomorphic and was used for genetic typing of M. hyopneumoniae isolates by PCR-sequencing without cultivation. This method appears to be easy and less expensive to use. However, it is not known if it can be applied to Ontario M. hyopneumoniae isolates. With support from the OMAFRA-AHL Disease Surveillance Program, we validated the p146 gene-sequencing typing method with Ontario, and a few out-of-province, field M. hyopneumoniae isolates.
The p146 PCR-sequencing had specificity and sensitivity similar to our current qPCR and can be used to perform PCR sequence typing directly from field samples without culture. About 190-210 nucleotide sequences were determined for each field strain. Using the standard of 4 nucleotide substitutions (~ 2% nucleotide substitution) as a new strain type, the 113 field strains identified at the AHL from 2009 to 2017 can be grouped into 11 p164 sequence types; most differed from strains described in Switzerland and France.
Most of the strains identified at the AHL in 2017 belong to a large cluster, indicating that the field strains might have been under selective pressure to become more homogeneous (Fig. 1).
Some strains from the same geographic location (based on postal code) and from different years had the same sequence types, indicating that the same strain types can circulate persistently in the field (Fig. 1). In contrast, different subtypes were also identified from similar geographic locations, indicating that a strain of the same subtype can spread widely (Fig. 1). Strains from lung tissue or oral fluid did not form distinct clusters, indicating no difference between these sample sources.
Our study indicated that the p146 PCR-sequencing is a useful typing method for Ontario M. hyopneumoniae strains, although its value in epidemiologic analysis needs further evaluation.
The AHL is offering this typing service for half-price ($40/sample) until May 1, 2018. AHL
Reference Mayor D, et al. Diversity of Mycoplasma hyopneumoniae in pig farms revealed by direct molecular typing of clinical material. Vet Res 2007;38:391-398.

Figure 1. Phylogenetic tree based on the serine repeat encoding region of the p146 gene of 113 M. hyopneumoniae field strains identified at the AHL. Strain names—first part is the year of isolation and case number; second part is postal code of the location origin of the samples; third part is the tissue origin (empty for lung tissue).
HORSES
A friendly reminder: serum amyloid A (SAA), a major acute-phase protein in horses, is routinely provided in all AHL comprehensive equine biochemistry profiles!
Kristiina Ruotsalo
Acute-phase proteins are produced by the liver in response to inflammatory or infectious stimuli. Increased hepatic production begins within 4-5 h of a stimulatory event, leading to increased serum concentrations that are a reflection of both protein production and peripheral catabolism. Major acute phase proteins, such as serum amyloid A (SAA) in horses, are present in negligible amounts (AHL reference interval: 0-20 mg/L) in healthy animals but increase rapidly and dramatically (often 1,000-fold) following an inciting event. Moderate acute-phase proteins, such as fibrinogen, are found in low concentrations in health, and increase modestly (10-fold) and more slowly (within 24-72 h), in response to inflammatory stimuli. The acute phase protein profile differs from species to species; with some proteins exhibiting the characteristics of a major acute phase reactant in one species, yet only negligible increases to inflammatory stimuli within another.
SAA is a major acute phase protein in horses. SAA is thought to play a role in cholesterol transport, platelet and neutrophil function, and modulation of inflammatory processes. Increased serum concentrations are found in horses suffering from a variety of disorders including viral and bacterial infections, inflammatory disease, colic, neoplasia, trauma and surgery. Foals have higher physiologic concentrations than adults for ~7 d after foaling. Increases in SAA occur much earlier in the course of disease than changes in total leukocyte count or fibrinogen, thus facilitating timely clinical intervention. Although infectious stimuli generally elicit a greater SAA response than inflammatory stimuli, the magnitude of increase is non-specific in nature, and does not support a particular etiology. SAA is rapidly cleared from circulation, therefore the high concentrations accompanying active inflammation or infection will decrease quickly once the eliciting stimulus has subsided, or successful treatment has been initiated. Serial SAA determinations may be helpful in gauging response to treatment and possibly as a predictor of recovery from illness. Relapse or clinical deterioration will result in sustained increases in SAA, which are then often accompanied by concurrent increases in moderate acute phase proteins such as fibrinogen. Recent studies have included evaluation of SAA concentrations in synovial and peritoneal fluids, and its use as a marker of performance in endurance horses; the reader is referred to the literature for additional details.

Mean concentrations of SAA (µg/mL), haptoglobin (Hp; mg/mL) and fibrinogen (Fb; g/L) in 19 horses undergoing elective surgery and 8 horses undergoing nonelective surgery.
From: Pollack et al. Effects of surgery on the acute phase protein response in clinically normal and diseased horses. Vet Record 2005:156;538-542.
SAA has been included in the AHL comprehensive equine biochemistry profile since 2010, and it has been an invaluable addition to the traditional markers of equine inflammation. AHL
References
Belgrade RL, et al. Assessment of serum amyloid A testing of horses, and its clinical application in a specialized equine practice. J Am Vet Med Assoc 2013;243:113-119.
Westerman TL, et al. Evaluation of serum amyloid A and haptoglobin concentrations as prognostic indicators for horses with colic. J Am Vet Med Assoc 2016;248:935-940.
COMPANION ANIMALS
Urine, the forgotten fluid….
Kristiina Ruotsalo
When it comes to laboratory submissions, one of the most frequently overlooked fluids is urine. This may be because urine can be difficult to collect if Fido just peed in the clinic flower bed prior to his appointment, or if Fluffy the cat would rather eat you than submit to a cystocentesis, but hopefully a brief overview of the potential information gained from a complete urinalysis will encourage increased collection and evaluation of this valuable material. Don’t forget the diagnostic utility of urinalysis applies to equine and bovine patients as well!
So what can a urinalysis tell us? Urinalysis can be used as a screening tool to evaluate the integrity of the urinary and other body systems. ‘Normal’ urinalysis results are valuable because they indicate that the physiologic processes governing the formation of urine (selective glomerular permeability, tubular resorption of some metabolites, and secretion of others) are functioning adequately, and may thus provide objective information to allow exclusion of the urinary system as a cause of clinical signs. The value of abnormal test results is obvious, but it should be pointed out that urinalysis may also indicate underlying disease processes earlier than serum tests - given the low renal threshold for bilirubin in most animals, bilirubinuria may be detected before hyperbilirubinemia in hemolytic and hepatobiliary disorders; mild proteinuria may be an early indicator of underlying renal disease or or hypertension.
A complete urinalysis includes determination of specific gravity, physical characteristics, chemical evaluation by reagent strip (dipstick), and sediment examination. It is important to assess all of these components as each supports the interpretation of the other, and to also consider how the sample was collected (cystocentesis vs catheterization vs free flow), as this can influence interpretation of results. Meaningful interpretation of physical urine characteristics (color, turbidity) and chemical test (protein, blood, pH, etc.) results is aided by knowledge of urine sediment results. Moderate proteinuria in the face of unremarkable urine sediment is most likely indicative of glomerular disease, whereas proteinuria accompanied by hematuria and pyuria is likely related to urinary tract inflammation or infection. Brown discoloration to urine may suggest hematuria or myoglobinuria, which would both result in a positive blood (heme) reaction on the reagent strip, but evaluation of urine sediment could be used to rule in/out hematuria, and further investigations of serum biochemistry could be utilized to determine if changes in muscle enzymes (CK, AST) are present, or if there is any evidence to support hemolysis.
Remember that reagent strip pads should not be used for specific gravity, leukocytes, or nitrites as these produce inaccurate results in domestic animals.
Knowledge of urine specific gravity (USG) by refractometry is a vital component of urinalysis and is needed for assessment of renal concentrating ability, particularly when animals are azotemic, polyuric, or oliguric. USG results should be interpreted with knowledge of the patient’s hydration status, current medications, and concurrently obtained serum biochemistry results as there are numerous disease conditions, in addition to renal disease, which can result in altered USG including diabetes mellitus, hypo- and hyperadrenocorticism, liver disease, urinary tract infections, and hypercalcemia, among others. Remember that in health USG can vary widely, depending upon hydration status.
Reagent strip analysis typically includes pH, protein, glucose, ketones, bilirubin, and blood.
pH is affected by both renal and extrarenal factors, including diet. Dogs and cats typically have a urine pH of 6.0-7.5, whereas herbivores range from 7.5-8.5. Changes in urine pH from expected ranges may indicate alterations in acid-base status, or the presence of urease-containing bacteria.
In health, there is very little protein present in urine, and most of that is albumin, which is the major protein detected by reagent stick pads. Protein results should always be evaluated in the context of the USG. Concentrated urine samples in dogs may have up to 1+ protein reactions in the absence of disease. Proteinuria in the absence of serum biochemistry changes may be an early indicator of renal disease, if post-renal conditions such as urinary tract infection are ruled out, or may help explain the cause of hypoalbuminemia noted on serum biochemistry. Alkaline urine may result in false-positive urine protein reagent stick results.
Glucose is typically all reabsorbed by the proximal tubules unless the tubular transport maximum is exceeded, which may be noted with both transient (excitement in cats) and persistent hyperglycemia (diabetes mellitus), as well as tubular disorders (Fanconi syndrome).
The reagent strip ketones pad detects primarily acetoacetate and acetone with a positive result indicating a need to search for disorders resulting in increased oxidation of fatty acids for energy (diabetes mellitus, starvation, and hypoglycemic disorders.)
The heme reagent stick pad detects heme-containing compounds (hemoglobin and myoglobin), which are not expected to be present in healthy animals. A positive result should prompt examination of the urine sediment for erythrocytes as can be found with inflammation, trauma, neoplasia, and coagulation defects. In the absence of intact erythrocytes, serum biochemistry and CBC findings should be examined to determine if hemoglobinuria or myoglobinuria are supported.
Bilirubin is not an expected finding in the urine of domestic mammals other than dogs. Concentrated urine of healthy dogs may produce a small-to-moderate positive bilirubin reaction in urine with a USG >1.040. Excessive bilirubin formation associated with hemolytic states, or impaired hepatobiliary excretion increases serum conjugated bilirubin concentrations which passes through the glomerular filter into the urine.
Examination of urine sediment allows the presence or absence of hemorrhage, inflammation, bacteriuria, cylindruria (casts in urine), crystalluria, and epithelial cells to be documented and integrated with the remainder of urinalysis findings and other laboratory data. Knowledge of expected sediment findings in health is vital to interpretation of urine sediment. In general, < 5 leukocytes and 5 erythrocytes/400X field (hpf) are expected; bacteria may be present in samples collected free flow but should not be identified in aseptically collected urine; casts may be found in limited numbers in healthy animals (typically hyaline casts), but the finding of granular or erythrocyte casts suggests renal tubular insult. Low numbers of transitional and potentially squamous epithelial cells can be found, depending upon sample collection method. Inflammation can induce cellular atypia; therefore, it is best to reassess transitional cell morphology following resolution of inflammation. Crystals can be found in healthy animals, with some species predilections such as calcium carbonate crystals in horses and low numbers of phosphate crystals in dogs and cats. Increased crystalluria may suggest the presence of certain pathologic states (e.g., infection) causing changes in either urine pH or ion concentration. The presence or absence of crystalluria is not a reliable indicator of the presence or absence of uroliths.
Hopefully this quick overview of the potential information derived from routine urinalysis has convinced you to collect a urine sample along with Fluffy’s CBC and biochemistry profile - the information is invaluable. AHL
 Focus on raw food
Focus on raw food