AHL Newsletter September 2018
Click here for a pdf copy of the September 2018 AHL Newsletter.
AHL pathologist update:
Beverly McEwen, DVM, MSc, PhD, Diplomate ACVP, has retired; last day August 29, 2018. Bev worked at the Veterinary Laboratory Services Branch of OMAFRA since 1993, and at the AHL since our inception April 1, 1997, as a Mammalian Pathologist and Surveillance Specialist, with a special interest and expertise in forensic pathology. Although she is retiring from the AHL she is teaching veterinary forensic pathology for the University of Florida’s online degree in veterinary forensic science. We wish Bev well in retirement.

Andrew Brooks, DVM, PhD, Diplomate ACVP, has worked at AHL-Kemptville as Laboratory Head and Veterinary Pathologist since May 2011, and is transferring to AHL-Guelph effective September 1, 2018, as a Veterinary Pathologist. Andrew will continue to serve as the AHL pathologist on the OAHN-Bovine network.

Emily Brouwer, DVM, DipPath, Diplomate ACVP, is on a one-year contract with the AHL in support of Ontario Animal Health Network activities, and provides gross and histopathology services for mammalian and avian species.
Heindrich Snyman will be taking up the position of Veterinary Pathologist/Laboratory Head, in Kemptville, in mid-September, 2018. Hein holds a BVSc from the University of Pretoria, 2008, a DVSc from the University of Guelph in 2013, and became a Diplomate of the American College of Veterinary Pathologists in 2013. Hein has broad experience with birds, exotics, domestic species, and most recently fish. We’re delighted that he is joining the AHL.

Update on the use of Premises Identification and lab submissions and the AHL Client Portal
Jim Fairles, Josie Given
Relating a laboratory submission and its outcomes to a location is important to monitor disease occurrence in livestock. Identifying submissions by farm of origin helps to transform AHL data into meaningful information for surveillance of disease problems for veterinarians, agricultural organizations, and producers.
AHL strongly encourages all veterinary practices to use PIDs on all submissions to any diagnostic laboratory. For participation in some Ontario programs (Ontario Swine Health, Ontario Animal Health Network projects) it is already mandatory. AHL already has clinics that regularly provide PIDs on sample submissions, and this year moving forward, we are emphasizing getting more clinics involved.
One of the tools that the AHL has rolled out is the AHL Client Portal in which clinic client data, including PID, can be stored for easy use on submissions. AHL is currently working with OMAFRA to make the process of obtaining client PIDs more streamlined. Please stay tuned for more information.
The client portal also streamlines submissions to the AHL and provides much more lab data information at your fingertips including easier searching and comparison capability.
If you are interested in using the client portal please contact the AHL! ahlinfo@uoguelph.ca
AHL Bovine Diagnostic Plans and sampling guides are available on the AHL website
Jim Fairles
Please see: https://www.uoguelph.ca/ahl/diagnostic-plans/diagnostic-plans-bovine
Diagnostic plans and guides are available for :
- Abortion
- Diarrhea
- Neurologic disease
- Respiratory
- Unexpected death / semi-comprehensive situations.
Formatting of the plans is scaled on the AHL website (as are all other pages) to be smart device friendly. The sampling guides can be downloaded for printing. Laminated versions are available from AHL for the cost of lamination ($5.00).
Thanks to all for looking at these. To get the maximum value from your laboratory submissions, correct sampling as well as a detailed history is a must! AHL
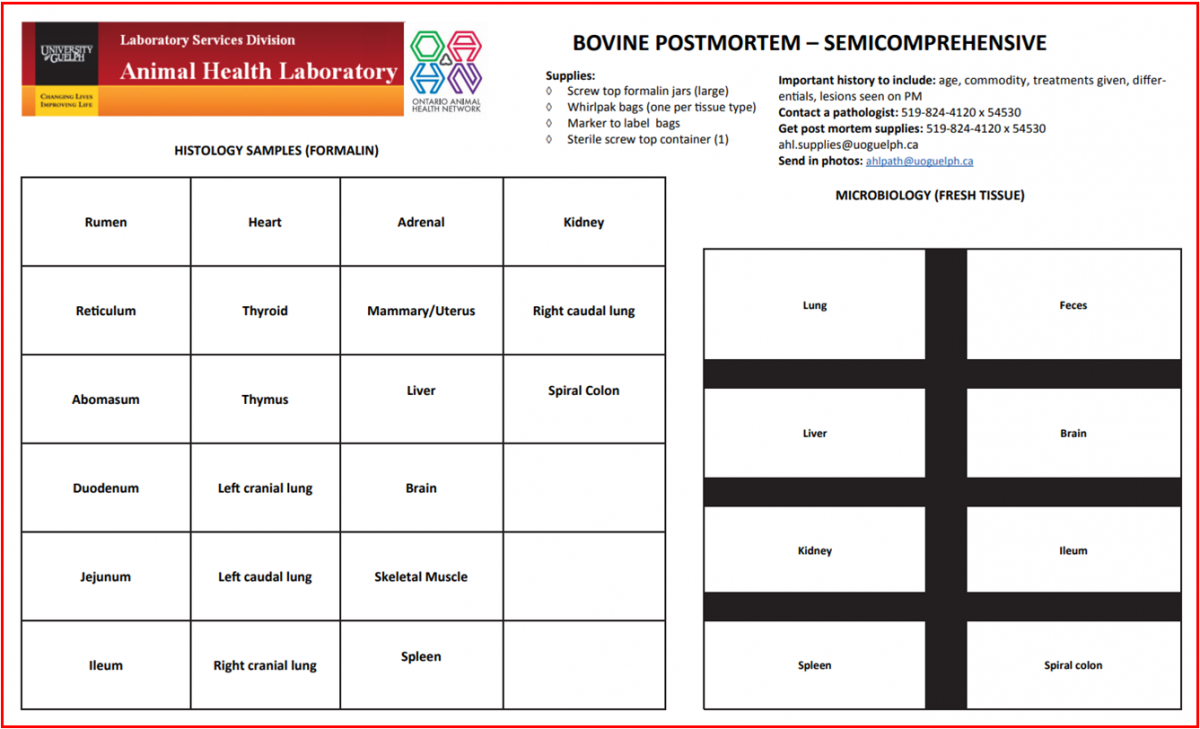
OAHN Update September 2018
Ontario Animal Health Network
Your comprehensive source for animal health information.
OAHN Update - September 2018
New OAHN Research Projects: We have just announced funding for projects related to improving surveillance by the OAHN Species Networks. The aim of these short-term projects (up to 12 months) is to support the overall goals of OAHN in terms of improving disease surveillance or contributing to protection of public health/food safety/market access. Each network has the opportunity to apply for a project up to $25,000, with applications accepted on a rolling basis. Money will be awarded based on projects presented and money available.
Approved projects: Equine - Potomac horse fever; Companion Animals - Best practices for infection prevention and control in small animal clinics; Poultry - Evaluating the prevalence of antimicrobial resistance in Salmonella, E. coli and Campylobacter isolates obtained from Ontario small poultry flocks. If you haven’t had a chance to check out the reports and resources for our previous round of OAHN projects, please find links to everything you need here: https://oahn.ca/news/oahn-disease-surveillance-projects/
Bee and Small Flock ListServs: We have just launched the Bee Veterinary ListServ. This ListServ provides a place for vets to ask case questions, and stay informed about important changes to bee medicine. For example, bees are included in the new regulations for antimicrobials in Canada. All beekeepers needing antimicrobials will require a prescription, the same as other species, as of December 1, 2018. The Small Flock ListServ was established nearly 2 years ago, and serves as a forum for veterinarians to get expert poultry health advice from recognized poultry veterinarians. If you are a veterinarian, and are interested in either of these Listservs, please email oahn@uoguelph.ca
Anti-Parasitics Table for Companion Animals: The OAHN Companion Animal Network created 2 tables that break down the different anti-parasitics available in Canada for dogs and cats, outlining what species the anti-parasitics are used for and the types of parasites they’re effective against. There is a simplified table for pet owners available here: https://oahn.ca/resources/anti-parasitics-table-for-dogs-and-cats-canada-2018/ and a veterinary version of the table available here (you must be registered and signed in to access the veterinary version): https://oahn.ca/resources/anti-parasitics-table-for-dogs-and-cats-for-veterinarians-canada-2018/

Updated Ticks and Lyme Disease Infographic: The OAHN Companion Animal Network has updated the tick map on its infographic: “Ticks and Lyme Disease in Ontario: What’s the Real Risk?” This infographic covers the types of ticks in Ontario, with risk areas for Ixodes scapularis, and provides a flow chart for a dog’s risk of getting Lyme disease if it’s bitten by a tick in Ontario. Access the updated Lyme Infographic by clicking here: https://oahn.ca/resources/ticks-and-lyme-disease-in-ontario-whats-the-real-risk-infographic-and-references/

Maximize Your Lab Dollars: The OAHN Equine Network has created a resource for veterinarians, outlining the top 10 ways to maximize your lab dollars. Tips cover best ways to package and submit histology and biopsy samples, labeling, use of proper containers, and much more. Access the tips here: https://oahn.ca/resources/top-10-ways-to-maximize-your-laboratory-dollars/
*New Reports*: We have recently published new veterinary and/or owner/producer reports for our Poultry, Companion Animal, Small Ruminant, Swine, and Equine networks. You can access the reports by going to https://oahn.ca and clicking on the Networks Reports tab. Note that you must be registered and signed in to view the veterinary reports, along with lab data and clinical impression summaries.
Do you ask your clients for their Premises IDs? Check out how fast and easy it is to do by registering your vet clinic: https://www.ontarioppr.com/home_en.html
RUMINANTS
Verotoxigenic E. coli in a goat herd.
Murray Hazlett, Jennifer Hanmore, Durda Slavic
A herd of dairy goats comprised of ~ 140 milking does, 130 doelings, and 160 kids experienced an outbreak of scours in the kids. All kids were housed in the same area in groups of 8-10 animals. This was the first outbreak of scours on the farm and there had been no recent introductions. There were no cattle on the farm. The diarrhea was usually very watery, although one animal that died had pasty/congealed feces with no blood noted. Most of the kids were ~ 10-d-old when the scours started, which lasted about 2 wk. Nine kids died. About 20% of the kids were affected, some with less severe clinical signs. The producer started the kids on an over-the-counter mixture of oral streptomycin/penicillin G with vitamin mixture and no additional animal became sick.
Two poor-doing 10-d-old kids were autopsied immediately after death with samples collected for histology and microbiology. Histologically, there were large numbers of both small bacilli (Fig. 1A) and, in some areas, gram-positive cocci could be seen adherent to enterocytes in both small intestine and colon sections. There was attenuation and exfoliation of luminal/superficial enterocytes, with occasional small aggregates of neutrophils in the superficial lamina propria. In one kid, there was edema with mild diffuse neutrophilic inflammation in the colonic submucosa.
A fecal floatation and PCR testing for bovine rotavirus and coronavirus were negative. E. coli was isolated in moderate numbers, along with Clostridium perfringens type A and Enterococcus durans. The E. coli was considered verotoxigenic, demonstrating genes for intimin and Shiga toxin type 1 as well as enterohemolysin. Testing for other genes, including for enterotoxigenic E coli, was negative.
Verotoxigenic E. coli has been identified in normal cattle, sheep, and goats, which can serve as reservoirs for this pathogen. In our case, it was felt to be significant based on the lesions seen in association with the large numbers of small gram-negative bacilli colonizing the brush border in areas of enterocyte exfoliation (Fig. 1B). We also identified Enterococcus durans in this case; its significance in diarrhea is uncertain – it has been associated with diarrhea in pigs and calves, and may also have contributed in our case.
A review of AHL pathology computer records (2008–2018) revealed 20 diagnoses of enteritis associated with E. coli in goats. Of these, where supportive bacteriology was done (12 cases), 3 were identified as enterotoxigenic E coli (ETEC - K99 +ve), 4 as enteropathogenic E coli (EPEC - with adhesion genes but no shiga toxin), and 5 as verotoxigenic E coli (VTEC - both intimin and a shiga toxin gene identified). VTEC +ve cases were 7-14-d-old. No other pathogens were identified or felt to be involved in the VTEC cases except for cryptosporidia in one case. AHL

Figure 1. A. Plump bacilli (arrow) attached to an exfoliating enterocyte. B. Gram (Brown and Hopps) stain showing gram-negative bacilli (arrow) heavily colonizing the surface of enterocytes.
SWINE
Tracheitis in pigs
Josepha DeLay, Murray Hazlett
In recent months, we have diagnosed tracheitis more frequently in swine cases submitted to the AHL (Figs. 1-3). Influenza A virus and possibly other undetermined viruses may contribute to tracheitis, although bacterial tracheitis predominates in most cases. This may reflect secondary bacterial infection and the subacute-to-chronic stage of the disease process. Trachea is infrequently included among samples received for microbiologic testing and histopathology. Please remember to include both fresh / frozen and formalin-fixed trachea among samples submitted for respiratory disease cases. AHL

Figure 1. Mild tracheitis. Tracheal mucosal epithelium is intact and mildly hyperplastic (bracket). Neutrophils are migrating through epithelium; lymphocytes and neutrophils in underlying lamina propria-submucosa.

Figure 2. Erosive tracheitis. The mucosal epithelial layer is very thin and epithelial cells are attenuated (arrow). Lymphocytes expand lamina propria-submucosa.

Figure 3. Severe ulcerative tracheitis. Mucosal epithelium replaced by fibrin, degenerate neutrophils, and bacteria (arrow). Edema, fibrin, and hemorrhage in lamina propria-submucosa.
AVIAN/FUR/EXOTIC SPECIES
Avian metapneumovirus subtype C in wild waterfowl in Ontario, Canada
Claire Jardine, Jane Parmley, Tore Buchanan, Larissa Nituch, Davor Ojkic
Reprinted from Transbound Emerg Dis 2018;65:1098-1102.
Avian metapneumovirus (aMPV) is an emerging poultry pathogen that has a significant economic impact on poultry production worldwide. The geographic range of the virus continues to expand, and wild birds have been implicated as reservoirs of aMPV that have the potential to spread the virus over long distances. Our objective was to determine the apparent prevalence of aMPV subtype C in wild waterfowl in Ontario, Canada. Wild waterfowl were captured in August and September, 2016, as part of routine migratory waterfowl population monitoring by the Ontario Ministry of Natural Resources and Forestry. Oropharyngeal and cloacal swabs were collected from each bird and placed together for aMPV testing using real-time RT-PCR. A total of 374 live wild birds from 23 lakes were sampled and tested for aMPV. Among all ducks tested, 84 (22%) were positive for aMPV. The proportion of samples that tested positive ranged from 0% in ring-necked ducks (Aythya collaris) and green-winged teal (Anas carolinensis) to 44% (8 of 18) in American black ducks (A. rubripes). Waterfowl positive for aMPV were found at 14 of 23 lakes in the study area and the percent positive at these 14 lakes ranged between 5% and 84%. Although subtype C aMPV has been detected in a variety of wild birds in North America, this is the first report of aMPV in wild ducks in Ontario, Canada. The high apparent prevalence, particularly in mallards and American black ducks (37 and 44%, respectively), suggests that these species may be important reservoirs of aMPV. Given the potential impact of aMPV on domestic poultry and the potential role of wild birds as reservoirs of the virus, further investigation of the geographic distribution, risk factors associated with aMPV carriage in wild waterfowl, and potential role of other birds in the epidemiology of aMPV in Canada is warranted.
Reovirus in Ontario: an overview since 2012
Emily Martin, Marina Brash, Davor Ojkic
Reovirus has been an ongoing issue in Ontario since 2012. Since the reovirus AHL Newsletter article in December 2012, there have been multiple outbreaks and the following is a summary of these outbreaks.
In the summer and fall of 2012, increased leg problems were noted in Ontario broilers (Fig. 1) including slight difficulty walking as well as splay leg or leg deformity. Flocks as young as 6 d were affected and losses were up to 16% as a result of culling. Histologic lesions included nonsuppurative synovitis or tenosynovitis and epicarditis. On PCR, closed hock samples were positive for avian reovirus (ARV). Clinical signs may or may not have been evident even if there were histologic lesions in the tendons/heart and if the reovirus PCR was positive. Clinically unaffected flocks could have been 300-400 g behind at processing (poor feed conversion) with increased ARV ELISA titers at processing. In order to further investigate this reovirus, 43 ARV isolates were genotyped using S1 segment sequencing, and 33 formed 2 distinct clusters: 40-50% different from ARV isolates in previous years (variant A and variant B). ARVs are transmitted vertically and horizontally (fecal-oral route), resistance to infection increases with age, they survive well in the environment, and control is by decreasing viral load (e.g. increased down time, total clean out, etc.).
Reovirus cases declined from 2012 to 2015, and cases spiked again in the summer of 2016 (Fig. 1). There was an increase in lameness cases (May-July), with an inconsistent number of cases and outcomes among Ontario poultry practitioners. Clinical signs included splayed legs, slipped tendons, tenosynovitis, and affected broilers were 7-30-d-old. Increased numbers of birds were unsuitable for loading at the end of the grow-out. Increased culling was the main concern, was highly variable; elevated mortality was less of an issue. Flocks of non-domestic chicks were at higher risk. When sequencing was performed on these isolates, both variant A and a new variant C were identified.
Overall, 8 new “variant” ARV groups that are associated with viral arthritis/tenosynovitis have been introduced since 2012. New viruses show low similarity to vaccines and historical ARVs. The origin of variant ARVs could not be determined, but suggested sources included other species or other countries.
In 2017 another spike in reovirus cases occurred (Fig. 1), but this time there was a mix of clinical signs in each case: variable age of onset, leg problems (lameness, leg deformities - splay legs, tenosynovitis), runting, stall in growth, percent of culls through life increased (culling rate 2-50%). More birds were unsuitable for loading at the end of grow-out. This outbreak was also confusing as in some cases it was difficult to establish whether there was vertical transmission or horizontal transmission or both. There was evidence of horizontal transmission in some broiler cases, however, the severity was not always proportional to the presence of source flock, there was a history of reovirus in the previous flock (barn carryover), there was close proximity to other infected barns (later age clinical signs) and affected barns were close to manure spreading. Geographically there was clustering of cases and there was geospatial risk to neighbors (both high-density broilers and breeders). In broilers, the total end-of-flock mortality ranged from close to normal (4%) to 80% affected, and there was depopulation of entire floors or barns. There were no clinical signs at breeder level (no changes in ELISA serology). Over the course of the 2017 outbreak, the source varied because early in year the higher risk flocks were from non-domestic egg source origin (i.e., eggs and chicks), whereas in the fall the case clusters were associated with imported or domestic breeder flock sources. Both horizontal and vertical transmission were noted.
In 2017 the actions taken for mitigation included stopping placements from affected breeder flocks, increased sanitation procedures at hatcheries (used quaternary and glutaraldehyde products), and destruction of associated hatch. However, broiler chicks from suspect breeders continued to be placed 6 wk after the first case was identified as the literature indicated that breeders develop immunity and reduce shedding at 3-8 wk. Testing of embryos was negative suggesting that there was low risk of vertical transmission. In addition, placements were grouped to minimize the number of potentially affected farms, and grower services worked with producers to monitor and identify affected flocks. The reovirus from broilers was typed as predominantly group D (50 isolates were 98.4-99.3% similar to the 2016 isolates and 99-99.8% similar to each other). A genotyping project was implemented to identify predominant genotypes, determine industry impact, examine geospatial mapping and epidemiology, and to investigate whether to pursue autogenous vaccine. It was decided to pursue autogenous vaccination with variant D strains, and the first chicks from vaccinated breeders will be available in the spring of 2019. AHL

Figure 1. Number of positive reovirus cases per year; 2018 is to date, August, 2018.
HORSES
From the horses’ mouth – equine oral lesions from AHL pathology records
Murray Hazlett, Josepha DeLay
Pathology diagnostic records from the AHL were searched from 1998 to May of 2018 for diagnoses of oral lesions in horses. Fifty-three cases were identified; 23 were identified as inflammatory, 21 as hyperplastic of neoplastic disease, 7 as trauma (dental attrition, bit trauma, tongue lacerations), and 2 as congenital anomalies (cleft palate).
Inflammatory disease usually involved ulceration and was often nonspecific (9 cases) or regarded as an incidental lesion in 3 cases (salmonellosis, hepatitis, colitis). Foreign-body material identified within the inflammatory response was suspected as the cause in 8 cases. Two cases were suspected as virally induced, one with equine cytomegalovirus isolated from tissue and from blood, and another suspicious because of histologic changes seen for a pox virus.
Ameloblastoma or ameloblastic fibroma was the most common dental tumor identified in 22 cases of hyperplastic or neoplastic disease (Table 1). Diagnoses were frequently qualified as “probable” because these tumors are rare and can be quite complex and inflamed. Odontomas (Fig. 1A) in domestic animals occur most frequently in horses, followed by dogs. Ameloblastomas (Fig. 1B) or ameloblastic fibromas were the most commonly diagnosed tumor of odontogenic epithelium (5 cases), and age was available in 4 of these horses, ranging from 3 to 22 y (mean = 12.5 y). AHL
|
Table 1. Equine hyperplastic or neoplastic oral masses diagnosed at the AHL, 1998 - 2018. |
|
|
Tumor |
Cases |
|
Ameloblastoma/ameloblastic fibroma |
5 |
|
Papilloma |
3 |
|
Odontoma/compound odontoma |
2 |
|
Melanoma |
2 |
|
Gingival fibrous hyperplasia |
2 |
|
Squamous cell carcinoma |
2 |
|
Cementoma |
1 |
|
Equine odontoclastic tooth resorption and hypercementosis |
1 |
|
Lymphoma |
1 |
|
Melanosis |
1 |
|
Fibroma |
1 |
|
Total |
21 |

Figure 1. A. A compound odontoma in a horse, with arrows showing enamel (attempts at tooth production; arrows).
B. An ameloblastoma in a 12-y-old Standardbred showing typical palisading epithelium (arrow).
COMPANION ANIMALS
Canine herpesvirus infection in a 5-wk-old puppy
Maria Spinato, Margaret Stalker, Davor Ojkic, Murray Hazlett
A 5-wk-old male Springer Spaniel puppy was submitted to the AHL-Guelph laboratory for postmortem examination. One other animal had died from this litter of 9 pups the preceding week. Clinical signs included conjunctivitis, dyspnea, lethargy, and weight loss prior to death. The bitch was fully vaccinated and mastitis had been ruled out. Bacterial pneumonia was suspected.
The puppy was mildly dehydrated and in good body condition with adequate fat stores. Approximately 10 mL of clear fluid was present within the thoracic cavity. Lungs were rubbery, edematous, and mottled light/dark-pink with indistinct parenchymal petechiae. Liver was bronze and contained miliary dark-red depressed foci throughout the parenchyma (Fig. 1A). Scattered petechiae were also present overlying the adrenal glands and serosa of the small intestine. Kidneys were mottled dark-red/purple and contained coalescing cortical hemorrhages on cut section. Spleen was dark red and minimally swollen. Hepatic and mesenteric lymph nodes were mildly to moderately enlarged and a few were markedly congested. Scattered linear mucosal hemorrhages were present within the small intestines and terminal colon. A preliminary diagnosis of generalized visceral petechial hemorrhages due to a suspected systemic viral or bacterial infection was made. Tissue samples were collected for microscopic evaluation and pooled viscera were submitted for PCR testing for canine distemper virus, canine adenovirus, and canine herpesvirus (CHV).
Histologic examination of liver revealed random coalescing foci of hepatocellular necrosis and hemorrhage lightly infiltrated by karyorrhectic neutrophils (Fig. 1B). Occasional eosinophilic intranuclear inclusion bodies were situated within surrounding viable hepatocytes (Fig. 1C). A kidney section also contained coalescing renal cortical hemorrhages surrounding small clusters of tubules undergoing acute necrosis. Rare suspicious eosinophilic intranuclear inclusions were observed in adjacent epithelial cells. Similar punctate foci of necrosis and hemorrhage were present in the lung, adrenal gland, spleen, abdominal lymph nodes, small intestine, and colon. The PCR test for canid herpesvirus 1 was positive (Ct 16.8). PCR tests for canine adenovirus 2, canine parainfluenza virus, and canine distemper virus were negative. Bacterial culture was not pursued. Final diagnosis was multifocal hepatic, pulmonary, adrenal, renal, and intestinal necrosis caused by CHV.
This case is unusual given the older age of the puppy. A search of AHL pathology records from 2007 to present found 14 diagnosed cases of CHV in puppies. All of these animals were < 3-wk-old except for this case (5-wk-old) and one 8-wk-old puppy that had CHV isolated in cell culture prior to PCR testing becoming available.
Canid herpesvirus 1 is a ubiquitous virus that is relatively fragile in the environment. Therefore, the source is thought to be activation of latent carrier status during stressful events such as parturition. Transmission is via direct contact from respiratory or genital secretions. Puppies > 2-wk-old are generally resistant to clinical disease. Several factors may increase the susceptibility to disease at an older age, such as seronegative status of the dam and cooler environmental temperatures that promote the survival and replication of the virus. Infected dams should develop protective immunity and subsequently produce healthy litters. AHL

Figure 1. A. Foci of hemorrhage in intestine, liver (arrow), and lung. B. Focus of necrosis and hemorrhage (arrow) with some neutrophilic reaction in liver. C. Typical eosinophilic intranuclear inclusion body in hepatocyte (arrow).
