AHL Newsletter March 2019
Click here for a pdf copy of the March 2019 AHL Newsletter.
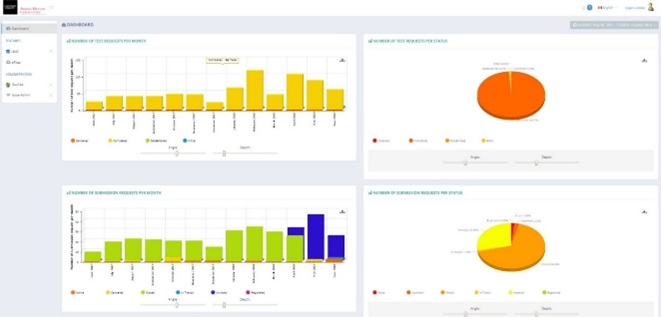
Premises ID and Client Portal
Jim Fairles, Josie Given
In partnership with OMAFRA, the Animal Health Laboratory will assist veterinary clinics and their clients in obtaining their Premises ID (PID). A single agreement with AHL is signed by the clinic stating that any premises information provided to the laboratory was done with the client’s consent. Together, we can work to quickly and efficiently get the PID numbers added to your client database. Once the client has been assigned a PID, a PPR certificate will be either emailed (preferred) or sent by mail to the owner of the premises.
AHL also now has a Client Portal for online submissions. Once your client PIDs are obtained, using the portal will :
Þ Standardize your lab submissions (spelling of owner names, PID, farm name, etc.).
Þ Track the progress of your cases, edit/cancel before the specimen is received at AHL.
Þ Allow advanced searching, design reports based on Animal ID, farm, owner, etc.
Þ Provide a case # prior to sending to AHL & track its progress as results are released.
Contact Josie Given: 519-824-4120 ext 54320, or jgiven@uoguelph.ca to setup training! AHL
Exotic, zoo, pocket pet, reptile, amphibian, and fish pathology at the AHL
The various AHL avian, exotic, and fur-bearing pathologists specialize in the gross and histopathology of non-domestic species, including mammals, pocket pets, birds, reptiles, amphibians, fish, and invertebrates. These animals are often affected by unique and species-specific diseases, necessitating a focused pathology service. The AHL has progressively established this specialized expertise and focus and we have tested a wide range of samples covering disease diagnosis and surveillance through to health checks. Given that these public display animals and pets are generally intimately associated with their caregivers, owners, and the general public, these animals also represent an important interface between animal and public health, and through laboratory testing and pathology screening act as a particularly critical indicator for potential zoonotic disease transmission.
Our clientele includes veterinary clinics, zoos and aquaria, other diagnostic laboratories, governmental institutions, and various universities. In September 2018, Dr. Heindrich Snyman joined the AHL in Kemptville. Dr. Snyman has a broad background in comparative pathology having worked with a variety of exotic, zoo, reptile, amphibian, fish, and invertebrate species, and his addition further expands the capacity of our comparative pathology service. AHL
Discontinuation of botulism testing
As of May 1, 2019, the AHL will discontinue mouse inoculation test (MIT) for detection of botulinum toxins in all animal species except poultry and wild birds. Our decision is based on anecdotal evidence since 2004 and review of AHL cases that were submitted for botulism testing in the last 3 years (70 in total). The MIT is able to detect toxins only in poultry and wild birds and no other species tested (i.e., equine and bovine). AHL
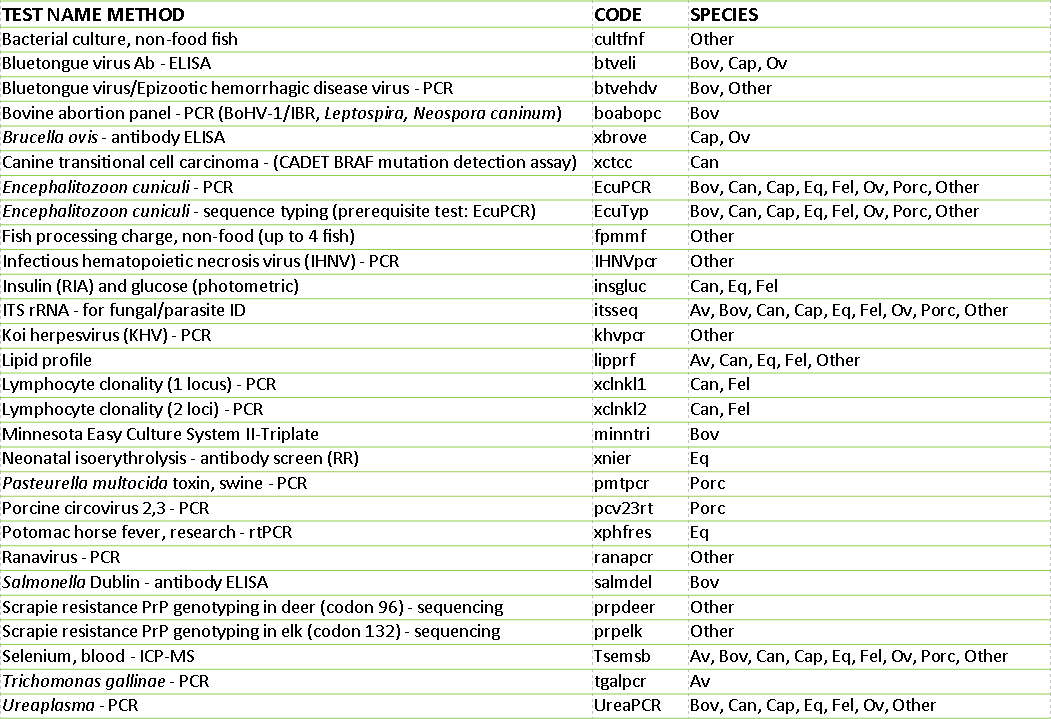
AHL new tests and services available in 2018
AHL Newsletter
March, 2019 - Volume 23, Number 1
Editor: Grant Maxie, DVM, PhD, Diplomate ACVP
Editorial Assistants: Helen Oliver, April Nejedly
The AHL Newsletter is published quarterly (March, June, September, December) by the Animal Health Laboratory, Laboratory Services Division, University of Guelph.
Its mission is to inform AHL clients and partners about AHL current activities, and laboratory-based animal disease events and disease trends. All material is copyright 2019. Ideas and opinions expressed herein do not necessarily reflect the opinions of the University or the Editor.
Articles may be reprinted with the permission of the editor and with appropriate credit given to the AHL Newsletter.
Mailing address & contact information:
Animal Health Laboratory
Laboratory Services Division, University of Guelph
Box 3612, Guelph, Ontario, Canada N1H 6R8
Phone: (519) 824-4120 ext. 54538; fax: (519) 821-8072
To receive an electronic copy of this Newsletter, please send your email address to us at holiver@uoguelph.ca
Contributors to this issue
- from the Animal Health Laboratory:
Marina Brash, DVM, DVSc, Diplomate ACVP
Emily Brouwer, HBSc, DVM, Diplomate ACVP
Hugh Cai, DVM, MSc, DVSc
Michael Deane, BA
Josepha DeLay, DVM, DVSc, Diplomate ACVP
Jim Fairles, DVM, MBA
Josie Given, BA
Murray Hazlett, DVM, DVSc, Diplomate ACVP
Mary Lake, AHT
Emily Martin, DVM, MSc, Diplomate ACPV
Davor Ojkic, DVM, PhD
Felipe Reggeti, DVM, PhD, Diplomate ACVP
Janet Shapiro, DVM, DipEqSurg, DipPath
Durda Slavic, DVM, PhD
Maria Spinato, DVM, DVSc, Diplomate ACVP
Margaret Stalker, DVM, PhD, Diplomate ACVP
Kate Todd, DVM
Other contributors:
Jeff Caswell, DVM PhD DACVP; Andrew Peregrine, BVMS, PhD, DVM, Diplomate EVPC, DACVM, Pathobiology, OVC.
Rex Crawford, DVM, Orangeville, ON
Tim Pasma, DVM, MSc, OMAFRA, Guelph, ON .
Our continued thanks to all of the non-author AHL clerical, technical, and professional staff who contribute to the generation of results reported in the AHL Newsletter.
Selected zoonotic pathogens and diseases from Ontario identified at the AHL, 2018
Murray Hazlett, Durda Slavic, Davor Ojkic, Hugh Cai
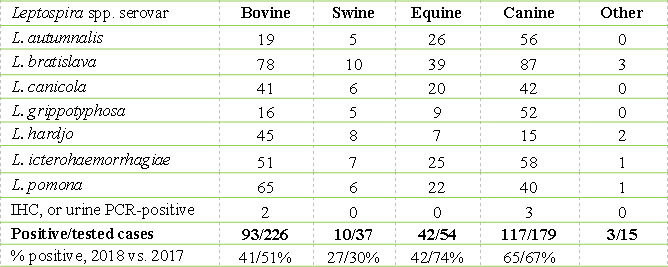
Many new, emerging, and re-emerging diseases of people are caused by pathogens originating from animals, or are shared between people and animals. The AHL plays a role in public health by identifying zoonotic pathogens in > 1,000 cases annually (Tables 1 and 2). The number and percentage of cases identified as positive for leptospirosis decreased in 2018 in all species, although the total number of submissions tested increased slightly with increased submissions from cattle, horses and swine, and decreased submissions from dogs. These are numerator data reliant upon submission biases to the diagnostic laboratory and cannot be regarded as population prevalence estimates. Monitoring programs are not included. AHL
Table 1. Number of cases with selected zoonotic pathogens isolated and/or identified at the AHL, 2018.
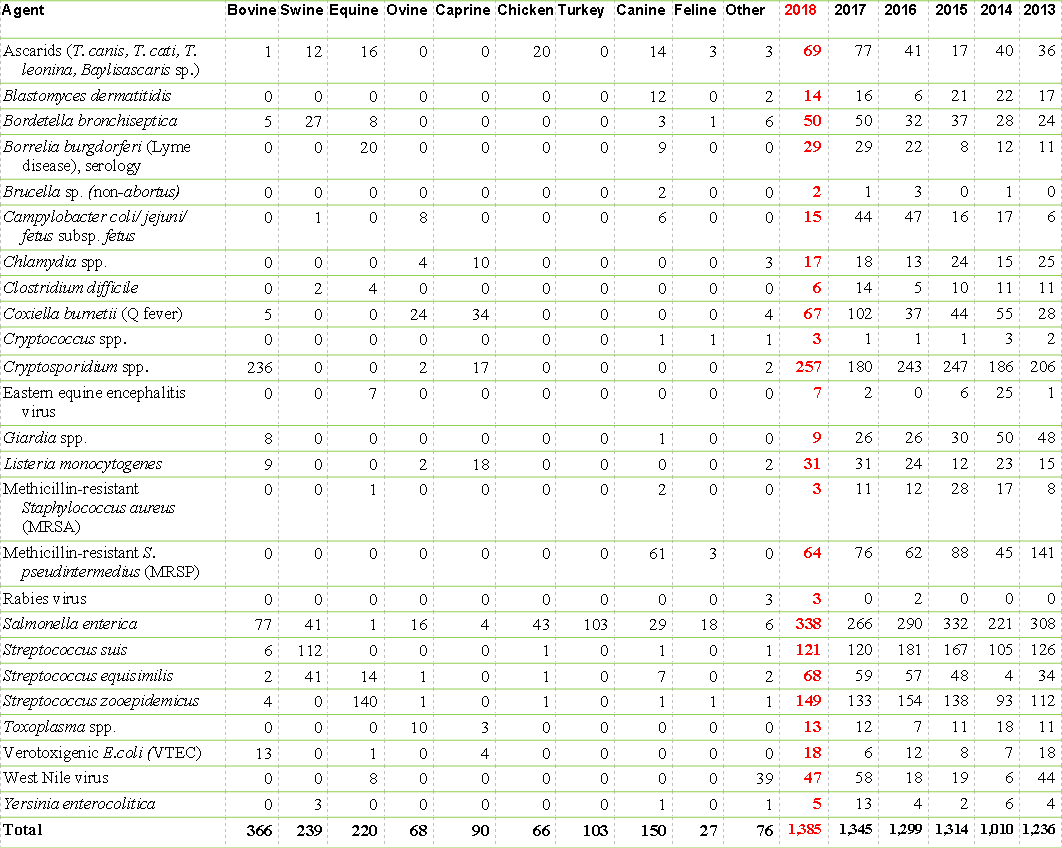
Table 2. Leptospira spp. seropositive, IHC-positive, or PCR-positive cases identified at the AHL, 2018.
Ontario Animal Health Network: OAHN Update - March 2019
 Your comprehensive source for animal health information.
Your comprehensive source for animal health information.
OAHN recently approved some new network projects:
The companion animal network put forward 2 initiatives; creation of a series of quick reference veterinary infographics on the topic of antimicrobial stewardship, and a research study to evaluate pathogen shedding in recently imported dogs, both pertinent topics in Ontario.
The wildlife network has a newly approved research project in the works, studying rodenticide exposure in non-target wildlife species. While the CWHC has noted several cases of rodenticide toxicity in non-target wildlife species, the true extent of rodenticide exposure in non-target Ontario wildlife is currently unknown.
The poultry network will be hosting a seminar on small poultry flock medicine and treatment as an initiative, on Apr. 6, 2019. Dr. Victoria Bowes of B.C. will provide lectures during the day, as well there will be laboratory sessions covering euthanasia techniques, diagnostic sampling methods, and postmortem findings in poultry. The registration for the session is full; it is apparent there is strong interest from practicing vets for education in this area.
If you haven’t had a chance to check out the reports and resources for our previous round of OAHN projects, please find links to everything you need here: https://oahn.ca/news/oahn-disease-surveillance-projects/
New Network Members
OAHN is pleased to welcome Dr. Cynthia Miltenburg as the OMAFRA Co-lead for the Bovine network. Cynthia graduated from the Ontario Veterinary College in 2010 and spent the next two years in a large animal practice in eastern Ontario. In 2012 she returned to the Ontario Veterinary College to pursue graduate studies with the Department of Population Medicine where she completed a Doctor of Veterinary Science degree in Ruminant Health Management with a research focus on managing transition cows for optimal immune function and metabolic health. Recently Cynthia has been coordinating the Dairy Health Management Continuing Education Program offered at the University of Guelph. We’re happy to have her on board! Please take the chance to say hello.
OAHN Presentations
Dr. Kate Todd presented an overview of successes and challenges of network collaboration at the National Farmed Animal Health and Welfare Council (NFAHWC) meeting in Ottawa, Ontario in November, 2018. The presentation was well received and is posted on the website under the publications tab (https://oahn.ca/publications) for reference.
New Podcast and Reports
The OAHN Equine Network has put together a new podcast with Dr. Janet Beeler-Marfisi. OAHN’s Dr. Alison Moore discusses Interpreting hemograms and biochemical profiles for the equine sports medicine veterinarian with Dr. Beeler-Marfisi. Listen to this podcast and all of our others here: https://oahn.ca/?s=podbean
The Q3 2018 reports for the networks have been posted to the OAHN site. Topics covered include:
* a handy summary of Immunodiagnostic tests for EPM, as well as a review of glanders (equine network)
* a review of Campylobacter hepaticus hepatitis in American conventionally housed layers (poultry network)
* an update (including images of associated lesions) on African swine fever (swine network)
* a case study on feline cuterebriasis (companion animal network)
* highlights of coldwater disease and updates to the in the livestock medicines act as it applies to aquaculture species (aquatic animal network)
We have lots of new reports, lab data, and resources. Be sure to check out OAHN.ca
RUMINANTS
Mycoplasma bovis antimicrobial susceptibility testing at the AHL
Hugh Cai, Jeff Caswell
Supported by OMAFRA and the Animal Health Strategic Investment (AHSI) program, the AHL examined temporal changes in the in vitro minimum inhibitory concentrations (MIC) of antimicrobials for 210 M. bovis isolates collected from 1978-2009, and found the following changes in MIC50 levels1 (Table 1):
1. The MIC50 levels of clindamycin, spectinomycin, and tulathromycin were low in the 1980s, increased in the 1990s to 8 μg/mL (clindamycin) and 32 μg/mL (spectinomycin and tulathromycin), then decreased again in the 2000s.
2. MIC50 levels for tetracyclines, tilmicosin, and tylosin tartrate were low in the 1980s, then increased in the 1990s and remained high.
3. Enrofloxacin, danofloxacin, and tiamulin MIC50s remained low (0.25 μg/mL) from the 1980s to 2000s.
MIC testing for M. bovis was implemented at the AHL in 2014. However, the tests were under-used for clinical isolates except for those from academic research. Although the number of field isolates tested is low, the test results indicated that the MIC for most antimicrobials had increased (Table 1). For example, MIC50s of a recent M. bovis isolate was 2 for chlortetracycline, 8 for oxytetracycline, >64 for tilmicosin, >32 for tylosin tartrate, >64 for tulathromycin, >16 for clindamycin, 2 for tiamulin, >1 for danofloxacin, >2 for enrofloxacin, 8 for florfenicol, and 64 for spectinomycin, which are mostly much higher than the isolates from the 1980s to 2000s.
Temporal changes in MIC levels of various antimicrobials show the importance of monitoring the susceptibility of mycoplasmas to antimicrobials. Fluoroquinolones, e.g., danofloxacin and enrofloxacin, are category I antibiotics (very high importance), and are considered essential for the treatment of serious bacterial infections in humans, and there is limited or no availability of alternative antimicrobials for effective treatment in case of emergence of resistance to these agents,2 and therefore they should not be used as first line antimicrobials. “The drug that has evidence-based efficacy and is in the category of least importance in human medicine should normally be selected as the first choice.”3 AHL
References
1. Cai HY, et al. Changes in antimicrobial susceptibility profiles of Mycoplasma bovis over time. Can J Vet Res 2019;83:34-41.
2. https://www.canada.ca/en/health-canada/services/drugs-health-products/veterinary-drugs/antimicrobial-resistance/categorization-antimicrobial-drugs-based-importance-human-medicine.html
3. https://www.canadianveterinarians.net/documents/pan-canadian-framework
MIC50 = the lowest concentration of an antimicrobial at which 50% of isolates are inhibited.
Table 1. AHL Mycoplasma bovis MIC50 (µg/mL) for bovine field isolates.

Chronic Erysipelothrix rhusiopathiae infection in lambs
Maria Spinato, Durda Slavic, Rex Crawford
Ten East Friesian lambs in a group of 80 animals developed ataxia and hindlimb weakness at ~ 2 wk of age. The lambs were treated with antibiotics and injected twice with selenium. A few animals improved slightly and there were no deaths. Three 5-wk-old intact male lambs were euthanatized and submitted to the AHL for complete postmortem evaluation. All 3 lambs had bilateral stifle synovitis characterized by increased amounts of cloudy synovial fluid and edematous synovial membranes; one lamb also had a small clump of organizing fibrin (pannus) within the joint. Vertebral columns were sectioned to examine bones and spinal cord. Two lambs had suspected diskospondylitis characterized by absence of L7-S1 intervertebral disks and associated irregular expansion of the intervertebral space which was filled with dark red fluid. Tails were not docked and omphalitis was not evident. No gross abnormalities were noted in viscera or the central nervous system. As an infectious etiology was suspected, joint and bone swabs were submitted for bacterial culture. Histologic evaluation was performed. Liver samples were submitted for a tissue mineral panel to rule out copper deficiency and selenium toxicity or deficiency.
Histologic examination of stifle synovia revealed marked lymphoplasmacytic villus hyperplasia (Fig. 1). Subsynovial stroma was infiltrated by a broad zone of lymphocytes, plasma cells and neutrophils. The affected intervertebral disk spaces were filled by an irregular mass of degenerate neutrophils and fibrin surrounded by hemorrhagic and organizing fibrovascular stroma. Focal chronic glomerulitis present in all 3 lambs was typified by dilation of urinary spaces by proteinaceous fluid, epithelialization and thickening of the parietal layer of Bowman capsules, and periglomerular fibrosis (Fig. 2). Amyloid deposits were present in the spleen of one lamb only. Hypomyelination was not observed in sections of brain, and there were no digestion chambers consistent with compressive myelopathy noted in the lumbar spinal cord segments. A single colony of Erysipelothrix rhusiopathiae was isolated from the joint swab of one lamb; no other significant bacterial pathogens were isolated from other swabs of affected joints or disks. Copper and selenium levels were within normal reference intervals.
Chronic polyarthritis and diskospondylitis caused by Erysipelothrix rhusiopathiae septicemia was diagnosed as the most probable cause of ataxia and hindlimb lameness in this group of affected lambs. Many cases of septicemia are subclinical, with infection only identified following localization in typical sites, including joints, kidneys, bones, brain, and heart valves. It can be difficult to confirm this etiology in chronic stages, because of the paucity of bacteria within affected tissues. Chronicity and/or antibiotic therapy may have played a role in the failure to isolate more than a single colony of E. rhusiopathiae from multiple lambs/swabs in this case, as also reported in other cases of chronic erysipelas polyarthritis in lambs and pigs. Even when synovial membrane scrapings rather than joint fluid swabs are cultured, only 1-2 colonies may be isolated.1 PCR is more sensitive for detection of bacteria in affected joints and tonsils of chronically affected lambs.1
Erysipelas septicemia is a not uncommon infection in the neonatal period, particularly in swine and sheep. Swine, birds and other wildlife are considered to be the usual carriers/shedders, and contamination of the environment is widespread. Persistent infection and associated antigenic stimulation are suspected to be the cause of lymphoplasmacytic synovitis and amyloidosis of the liver and spleen. Endocarditis is also an occasional sequela diagnosed in juvenile sheep. AHL
Reference:
1. Ersdal, C, et al. Acute and chronic Erysipelothrix rhusiopathiae infection in lambs. Vet Pathol 2015;52:635-643.

Figure 1. Marked proliferation of stifle synovium with extensive lymphoplasmacytic infiltrates.
Figure 2. Chronic glomerulitis with epithelialization and thickening of Bowman capsules and periglomerular fibrosis in affected glomeruli (arrows). Compare to normal glomeruli (stars).
Unusual intestinal lesion in a goat
Jan Shapiro
Formalin-fixed tissues were submitted from a 4-y-old male Boer goat from a herd of 200. The goat had been losing weight for a few weeks, and had not responded to a course of antibiotics and dewormers. A field postmortem showed that the goat was emaciated, and had 2 segments of the jejunum that were stenotic because of intramural thickening by white firm tissue (Fig. 1), and other intestinal segments had clusters of raised white foci on the serosa. The wall of the stenotic segments of jejunum was 5-6 times thicker than the unaffected segments.
Jejunal histopathology confirmed the presence of a very scirrhous intramural tumor which appeared to originate in areas of the mucosa with dysplastic crypts. Tumor cells formed acinar or glandular structures which infiltrated the full thickness of the jejunal wall, and onto the serosal surface, and were surrounded by marked fibroblastic response (Fig. 2). Tumor clusters were seen in a few vascular structures. The tumor diagnosis was intestinal adenocarcinoma.
Histopathology of other sections of the intestine and the ileocecal lymph node revealed underlying granulomatous enteritis and lymphadenitis typical of paratuberculosis (Johne’s disease), which was confirmed by a positive PCR test for Mycobacterium paratuberculosis. Paratuberculosis was likely a major factor in emaciation, given that intestinal adenocarcinoma in small ruminants is often an incidental finding at slaughter.
Unlike sheep, intestinal adenocarcinoma is not common in goats. The mid-jejunum is a common site, forming dense white polypoid or intramural masses extending from 0.5 to several cm along the gut segment, and causing annular constriction of the lumen. Weight loss and sometimes ascites are clinical signs. The tumor is highly infiltrative and can metastasize to local nodes and distal sites, as well as spreading through the peritoneum. In sheep in New Zealand, where the disease is common, there may be an association with exposure to bracken fern or other unidentified carcinogens, heavy use of certain fertilizers, and pastures with the weed Cyanosaurus cristatus. There may also be a genetic predisposition. The risk factors for this tumor in goats are not known, likely because of the small number of reported cases. AHL
References
Munday JS, et al. Tumors of the alimentary system. In: Tumors in Domestic Animals. Meuten DJ, ed. 5th ed. John Wiley, 2017:564-568.
Uzal FA, et al. alimentary System. In: Pathology of Domestic Animals, Maxie MG, ed. 6th ed. Elsevier, 2016, vol 2:103-104.

Figure 1. Section through formalin-fixed jejunum showing segmental transmural thickening.

Figure 2. Histologic section through stenotic jejunal segment, arrows showing tumor infiltrates (mucosa at bottom of photo).
SWINE
OAHN-swine project: erysipelas in Ontario swine
Josepha DeLay, Durda Slavic, Tim Pasma, Jim Fairles
Erysipelothrix rhusiopathiae septicemia (‘erysipelas’) remains a concern in swine despite continued routine sow vaccination in many herds. In clinical impression surveys carried out by the Ontario Animal Health Network (OAHN), Ontario swine veterinarians have indicated an increased frequency of erysipelas over the past 3 years. Cases of suspected erysipelas are often diagnosed clinically based on distinctive, nearly pathognomonic, rhomboid skin lesions and typically successful response to antibiotic therapy. As a result, these cases are infrequently submitted to the Animal Health Laboratory (AHL) for diagnostic confirmation.
Swine erysipelas may affect all age groups. Typical skin lesions may not be seen in all acute cases, and sudden death may be the primary complaint. Chronic arthritis and lameness, and vegetative valvular endocarditis may be sequelae in surviving pigs. Asymptomatic carrier pigs shed the organism in oronasal secretions and feces; in acutely infected animals, the organism may also be shed in urine.
The multiple known strains of E. rhusiopathiae vary in virulence, and this variation could potentially explain recent clinical reports of an increase in swine erysipelas. Isolation of E. rhusiopathiae is usually straightforward in samples from filtering organs (spleen, liver, lung, kidney, lymph node; also affected skin) of acutely affected, untreated animals. As with most infectious diseases, selection of appropriate individual animal and tissues for sampling is the key to reaching an accurate, successful diagnosis.
Since 2015, 5 cases of E. rhusiopathiae septicemia have been submitted to the AHL. The cases involved nursing piglets (2 herds, including 1 organic herd), sows (1 herd), and grow-finish (GF) pigs (2 herds). Typical skin lesions were described in 1 of the GF herds, but not in other cases. A sudden increase in mortality was described in the nursing piglet cases, with a concurrent increase in stillborn and weak neonates in 1 herd. In all cases, E. rhusiopathiae was isolated in large numbers from various filtering organs (spleen, liver, lung, kidney, lymph node), as well as from skin and meninges in some animals.
E. rhusiopathiae is a zoonotic pathogen, and education of producers and barn staff in this regard is important. Localized cutaneous lesions (‘erysipeloid’ or ‘pork finger’) are most common, although systemic illness and endocarditis occur in some people, with potentially fatal outcome. Notably, the disease known as ‘erysipelas’ in humans is a separate and distinct condition, and is caused by Streptococcus spp.
In response to swine practitioners’ identification of increased erysipelas cases in the Ontario herd, the OAHN Swine Network is supporting a project to further investigate and characterize E. rhusiopathiae isolates from clinical cases and from abattoir samples.
Veterinarians with suspected erysipelas cases are encouraged to contact Dr. Tim Pasma, Ontario Ministry of Agriculture, Food, and Rural Affairs (OMAFRA) to enroll in the project (tim.pasma@ontario.ca).
For enrolled herds, fresh or frozen samples from spleen and lung of affected pigs should be submitted to the AHL for bacterial culture. AHL
Chlamydia spp. and reproductive issues in swine
Josepha DeLay, Jim Fairles, Hugh Cai
Recent reports in peer-reviewed publications and in the lay press have suggested an association between sow infection with Chlamydia spp. (primarily C. suis) and poor reproductive performance. A definitive, causal relationship between Chlamydia spp. and poor conception or pregnancy loss remains to be proven. The Animal Health Laboratory (AHL) is developing a PCR assay for C. suis, in order to evaluate the presence of the organism in the Ontario herd, and to investigate a potential link between infection and reproductive failure. The test is currently available as an external (send-out) test. Stay tuned for updates on this topic in future editions of the AHL Newsletter. AHL
AVIAN/FUR/EXOTIC SPECIES
Infectious bronchitis virus testing update
Davor Ojkic, Marina Brash, Emily Martin, Emily Brouwer
The number of samples submitted to the Animal Health Laboratory for infectious bronchitis virus (IBV) testing has increased significantly over the last 10 years (Fig. 1). Intensified testing began with incursion of variant IBVs into Ontario’s commercial chicken flocks of various commodities in 2012 and 2013, with IBV 4/91 the most frequently detected IBV strain (Fig. 2) at that time. This strain was reported to be endemic in Europe, Africa and Asia, and at this time is also present in Quebec, but has still not been reported in the USA.
As other variant strains were introduced into Ontario chicken flocks, the demand for IBV testing continued to climb, reaching almost 1,800 samples in 2018. Consequently, the number of requests for IBV genotyping also increased, peaking at > 300 samples genotyped in 2017 and 2018 (Fig. 1). In 2015, the number of IBV 4/91 infected flocks was trending downward, but IBV re-emerged in late 2015 causing high production losses in Ontario poultry flocks. With genotyping, a new IBV strain related to a strain DMV/1639/11, first detected in 2011 in broiler flocks on the Delmarva (DMV) peninsula, was identified in Ontario (Fig. 2). In early 2019, this IBV strain continues to be identified in commercial flocks, however the clinical impact has been reduced through the implementation of novel vaccination strategies using existing IBV vaccines in conjunction with good management and heightened biosecurity.
In addition to the IBV 4/91 and DMV strains, US variant strains including California 1737/04 and Pennsylvania Wolg/98 also continue to circulate in Ontario and are associated with production losses and elevated mortality but at a lower frequency. PA/Wolg was first detected in Ontario in 2000-2002 and CA/1727/04 in early 2012.
The ability to genotype IBV strains is critical to the management of this disease given that multiple strains are circulating at any one time in the province and, based on Ontario’s track record, there is always a risk of new incursions. AHL

Figure 1. Number of samples tested for IBV by PCR and genotyped at the AHL from 2009-2018.
Figure 2. Genotypes of IBVs detected at the AHL from 2012-2018.

HORSES
Alcohol and Gaming Commission of Ontario (AGCO) Death Registry / Equine Incidents in Ontario Racing program: 2003 - 2018 postmortem summary
The Alcohol and Gaming Commission of Ontario (AGCO; formerly the Ontario Racing Commission, ORC) continues in its proactive approach to advance racehorse welfare and safety of human and animal participants. In 2003, Ontario became one of the first North American racing jurisdictions to require mandatory reporting of racehorse deaths, in order to monitor, research, and improve knowledge of why these events occur. Postmortem (PM) exams conducted at the Animal Health Laboratory (AHL) through the AGCO Death Registry (DR, 2003-2016) and Equine Incidences in Ontario Racing (EIOR, 2016-current) programs continue to provide comprehensive data regarding the causes of morbidity and mortality in racehorses in this province. To date, PM has been carried out on 1,125 horses through these programs (Table 1). Annual variation in the number of PM cases reflects the discretionary requirement for PM on the part of the Registrar of AGCO.
A summary of significant PM findings is provided in Table 2. A comprehensive review of AGCO PM cases was conducted in 2015 as part of a separate retrospective study and as a result, some cases have been reclassified from results presented in previous editions of the AHL Newsletter. Results of the study were published in the July 2017 edition of the Journal of Veterinary Diagnostic Investigation.1
Since 2015, computed tomography (CT) of fractured and contralateral limbs has been carried out on select DR and EIOR postmortem cases through collaboration with the Diagnostic Imaging section of the Ontario Veterinary College Health Sciences Center. The goal of this in-depth examination is to identify pre-existent lesions, primarily in bone, that contribute to catastrophic fractures. The procedure was continued in 2018, with CT imaging of all 29 limb fracture cases submitted for PM exam. Pre-existent lesions in bone were identified by CT and considered potentially predisposing to fracture in 18 of 29 (62%) cases.
Exercise-associated sudden death continues to be of special concern in the racing industry. At the AHL, a modified in-depth PM protocol is used in the evaluation of these cases, with special emphasis on cardiovascular and respiratory systems. In 2018, the cause of death was investigated in 5 horses that died while exercising. Significant pulmonary hemorrhage was evident in 4 horses, and no cause of death was identified in 1 horse. Among all sudden death cases from 2003-2018, significant pulmonary hemorrhage was identified in 88 of 177 (50%) horses. The cause of death in such cases is often attributed to exercise-induced pulmonary hemorrhage (EIPH), although the pathogenesis of pulmonary hemorrhage in these horses is not well understood. In 41 of 177 (23%) exercise-associated sudden death cases from 2003-2018, no potentially fatal lesions were identified and the cause of death remained undetermined. It has been speculated that exercise-associated cardiac dysrhythmia, leading to acute heart failure and pulmonary hypertension, may be the underlying cause of death among many of these horses, and may also contribute to pulmonary hemorrhage in these animals.2 Typically, no morphologic lesions are detected in the heart as a cause or result of fatal ventricular dysrhythmia, and the diagnosis cannot be confirmed based on PM findings.
Summaries of postmortem submissions to the Animal Health Laboratory under this program and diagnoses by body system for these cases are provided in the following tables. AHL
References
1. DeLay J. Postmortem findings in Ontario racehorses, 2003-2015. J Vet Diagn Invest. 2017;29:457-464.
2. Physick-Sheard PW, McGurrin MKJ. Ventricular arrhythmias during race recovery in Standardbred racehorses and associations with autonomic activity. J Vet Intern Med 2010;24:1158-1166.
Table 1. Breed distribution of AGCO Equine Incidents submissions to the AHL, 2003-2018.

Table 2. Significant postmortem lesions identified in AGCO Death Registry submissions by body system, 2003-2018.

Dicrocoelium dendriticum in the liver of a miniature horse
Murray Hazlett, Margaret Stalker, Mary Lake, Andrew Peregrine
Following a humane society investigation, an emaciated miniature horse was submitted to the AHL for postmortem. It had no fat stores and was estimated to be relatively young. It had been frozen, however routine histologic examination revealed areas in the liver with moderate periportal fibrosis. A cross-section of a small fluke could be seen, along with some operculate eggs. We were able to recover a fluke following fine dissection of the liver and bile ducts, and identified it as Dicrocoelium dendriticum based on morphology as well as characteristic eggs that were released from the specimen (Fig. 1).
Dicrocoelium dendriticum is a small fluke that usually infects sheep, cattle, and goats as well as camelids. It has a unique life-cycle involving land snails and ants. Briefly, infected snails release infected “slime balls” that are eaten by ants, in which the parasite develops in the nervous system. At temperatures < 15oC, the parasite causes the ant to stay on the tips of grass blades where it can be eaten (see the 2 references below for more of this fascinating story).1,2
Dicrocoelium dendriticum is very common in Europe. This trematode is thought to have been introduced to Canada in the 1930s, likely from Europe1 and is now often recognized in Ontario; lesions are commonly seen in sheep and goat livers in slaughter plants (Alexandra Reid, OMAFRA, pers. comm.). There are documented cases of D. dendriticum in horses in Europe, Asia, and Africa, however it has not been reported in a horse in North America before our recent report1 in the Canadian Veterinary Journal. AHL
References
1. Hazlett M, et al. Hepatic Dicrocoelium dendriticum infection in a miniature horse. Can Vet J 2018;59:863-865.
2. Stalker MJ, et al. Hepatic infection with the lancet fluke, Dicrocoelium dendriticum, in an Ontario sheep flock. AHL Newsletter, September 2007:22–24.

Figure 1. A. Dicrocoelium dendriticum cross section in remnants of a bile duct. Eggs can be seen in the uterus of the trematode (arrows). H&E. B. More longitudinal orientation of a partial D. dendriticum in a bile duct. The oral sucker is visible (arrow). H&E. C. Periportal fibrosis (arrows) including necrotic cellular debris in a larger duct (large arrow). H&E. D. Eggs of D. dendriticum released from the dissected frozen, unfixed, liver tissue. Wet mount. E. Autolyzed body of recovered intact D. dendriticum. Wet mount. Reprinted with permission from the Canadian Veterinary Journal (Can Vet J 2018;59:863-865).
COMPANION ANIMALS
Myelodysplastic syndrome progressing to acute myelogenous leukemia in a dog
Felipe Reggeti, Emily Brouwer
A 6-y-old Shetland Sheepdog was presented to the OVC with history of pancytopenia (Table 1). There were no atypical cells noted in the blood smear. SNAP 4Dx and direct Coomb’s were negative. Coagulation profile was unremarkable. Considering multi-lineage unexplained cytopenias, bone marrow (BM) examination was performed.
BM smears showed predominance of erythroid cells (Fig. 1), with a markedly decreased granulocytic-to-erythroid ratio (1:10). There was an increased proportion of rubriblasts, whereas metarubricytes and polychromatophils were observed in very low numbers. Binucleate rubricytes, mitoses, and irregular nuclear shapes were present. A BM core biopsy was > 95% cellular (Fig. 1, inset). These findings indicated erythroid hyperplasia, dysplasia and ineffective erythropoiesis (dyserythropoiesis), compatible with myelodysplastic syndrome (refractory anemia with excess blasts) or precursor-targeted immune-mediated anemia.
The patient improved after 2 wk on cyclosporine and prednisone (Hct 0.17 L/L; platelets 113 x 109/L; neutrophils 11.3 x 109/L; reticulocytes 306 x 109/L). Unfortunately, 2 mo after initial presentation, the dog became very sick, with severe non-regenerative anemia (Hct 0.10 L/L), leukopenia, and thrombocytopenia. Given progressive deterioration and inconsistent response to treatment, euthanasia was elected.
On postmortem examination, there was hepatomegaly, splenomegaly, and discolored red bone marrow in humeri and femurs. Microscopically, 80% of the cells in the BM were large myeloblasts with 44 mitoses/10 hpf and myeloid-to-erythroid ratio 8:1; ~ 90% of the splenic parenchyma was effaced by neoplastic myeloblasts (Fig. 2), and similar cells were noted within the hepatic sinusoids (Fig. 3). Histopathology was diagnostic for acute myeloid leukemia.
Myelodysplastic syndrome progressed in this dog to acute myeloid leukemia. In humans, myelodysplastic syndrome consists of a heterogeneous group of myeloid neoplasms characterized by cytopenias, ineffective hematopoiesis, morphologic abnormalities, and genetic instability leading to acute myeloid leukemia in ~ 30% of patients. AHL
Table 1. Hemogram of a Shetland Sheepdog with myelodysplastic syndrome, on presentation.


Figure 1. Very cellular marrow smear, with predominance of erythroid cells. Inset: Very cellular bone marrow core biopsy.

Figure 2. Splenic parenchyma effaced by neoplastic myeloblasts.

Figure 3. Hepatic sinusoids contain neoplastic myeloblasts.
Baylisascaris procyonis encephalitis in a dog
Murray Hazlett, Hugh Cai
Baylisascaris procyonis - the raccoon round worm - is found very commonly in raccoons in southern Ontario. The larval form of B. procyonis is well recognized as a cause of visceral larval migrans in humans and dogs. A recent submission of a 14-wk-old puppy from the metropolitan Toronto area was diagnosed with encephalitis as a result of migration of B. procyonis larvae1 (Fig 1). The dog had suffered from a 1-wk period of tremors and hypermetria followed by increasing neurologic deficit and uncontrolled paddling, and had eosinophilia. Diagnosis was confirmed by the morphology of the parasite as well as PCR testing (which is now an available test at the AHL. Baylisascaris procyonis - PCR. Code = bpropcr).
We suspect that visceral larval migrans caused by this parasite is underdiagnosed, with some cases being non-fatal, depending on the number of larvae involved. It is important to realize that eggs are found in large numbers in areas where raccoons defecate (“raccoon latrines”) and can survive for years in the environment2. Most human cases are in young children due to play habits and poor hand/mouth hygiene. Both adult and juvenile raccoons can shed eggs, in one study shedding up to 23,600 and 228,000 eggs per gram of feces respectively.3 AHL
References
1. Hazlett M, et al. Neurologic Baylisascaris procyonis infection in a young dog. Can Vet J 2018;59:1325-1328.
2. Kazacos KR. Baylisascaris procyonis and related species. In: Samuel WM,et al., eds. Parasitic Diseases of Wild Mammals. 2nd ed. Ames, Iowa: Iowa State Univ Pr, 2001:301–341.
3. Snyder DE, Fiktzgerald PR. Contaminative potential, egg prevalence, and intensity of Baylisascaris procyonis-infected raccoons (Procyon lotor) from Illinois, with a comparison to worm intensity. Proc Helminthol Soc Wash 1987;54:141–145.

Figure 1. Baylisascaris procyonis larvae (arrows) migrating in cerebral cortical white matter. H&E.