Proper fixation of surgical biopsies in 10% neutral buffered formalin is necessary to preserve tissue detail and ensure optimal histopathology results. Maintaining a 1:10 tissue: formalin ratio during fixation is very important for adequate fixation and preservation of histologic features of the biopsy. Evaluation of the entire biopsy is necessary for assessment of surgical margins. For small excisional and incisional biopsies, including punch biopsies, this is easily accomplished using standard 60 mL formalin vials provided by most diagnostic laboratories. In contrast, fixation of large excisional biopsies presents a logistical challenge in a clinical practice setting, and margin evaluation has to be selective, especially for larger biopsies. Pathologists assessing the tissue before slide preparation will target the most suspect areas (closest margins) of the tissue for sectioning.
Incorporating a few simple variations in routine procedures will allow adequate tissue fixation while avoiding the hazards and inconvenience associated with shipment of large volumes of formalin.
Option 1. Completely fix oversize excisional biopsies in a large volume of formalin, then remove and package for transport to the diagnostic laboratory (Figures 1-3).
Advantage: best fixation and specimen for laboratory assessment, as the biopsy remains intact.
Disadvantage: procurement, handling, and disposal of larger volumes of formalin are problematic.
|
|
|
|
|
Figure 1 |
Figure 2 |
Figure 3 |
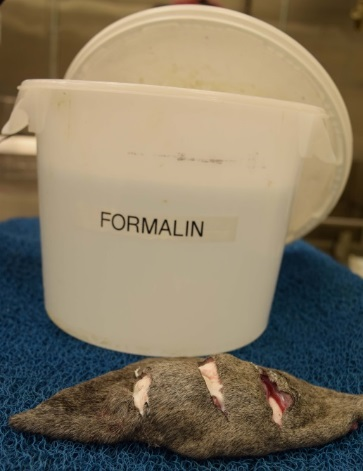
Figure 1. Make several parallel, partial-thickness incisions through the biopsy (‘breadloafing’) to allow formalin exposure at the center of the biopsy. Using a large covered container, immerse the biopsy in sufficient formalin to maintain a 1:10 tissue: formalin ratio.
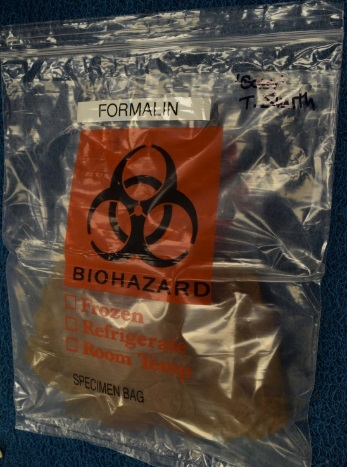
Figure 2. After 48-72 hours fixation, remove the biopsy and wrap in paper towels soaked with formalin. The biopsy may also be placed directly in a sealed plastic bag, without paper towel. Be sure to handle formalinized tissues in a well-ventilated area, and limit exposure time.
Figure 3. Fixed biopsy in sealed, labelled plastic bag, ready for transport to the diagnostic laboratory. Place the bag inside a second, outer sealed plastic bag.
Option 2. Section the large biopsy and fix in separate labelled sample containers (Figures 4 and 5).
Advantage: good for medium-sized biopsies that can be partitioned and fit into multiple small formalin jars.
Disadvantage: additional preparation (margin inking, diagram preparation) is required for meaningful results. Fatty tissues especially may be difficult to quarter and retain useful margins.
Labelling of biopsy margins immediately after surgical excision, using tissue-marking dye, is recommended for all biopsies. However for this method of oversize sample handling, labelling of surgical margins is imperative to allow accurate reconstruction and trimming of the biopsy at the diagnostic laboratory. Following application of surgical ink and blotting to dry the sample, the biopsy is quartered or otherwise sectioned to sizes that can be accommodated in the formalin containers available. As always, it is important to maintain a 1:10 tissue: formalin ratio. A labelled drawing or image of the biopsy must accompany the submission to the diagnostic laboratory, and each container must have a corresponding label, to allow proper orientation of the biopsy segments. In this way, surgical margins may be accurately identified and evaluated by the pathologist.
|
|
|
|
Figure 4 |
Figure 5 |
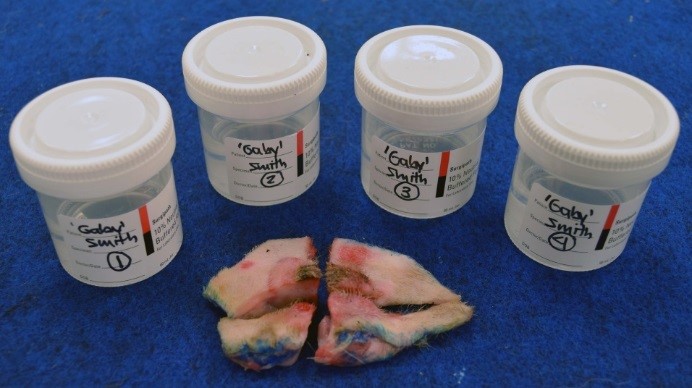
Figure 4. Large excisional biopsy divided into segments that can be accommodated in formalin containers available and easily reconstructed at the diagnostic laboratory for trimming. Note that surgical margins were inked with tissue dye PRIOR to sectioning.
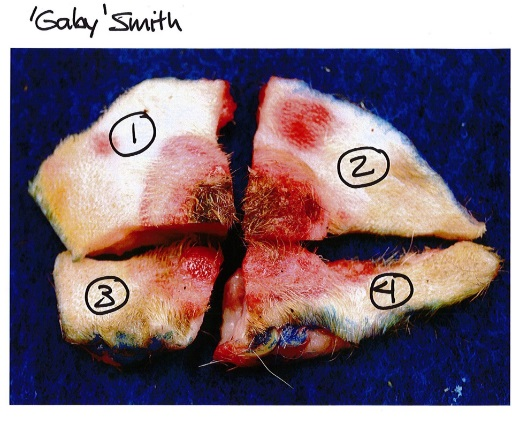
Figure 5. Labelled photo of large biopsy divided for fixation, to accompany submission to the diagnostic laboratory. Numbers correspond to the labelled sample vials containing each biopsy segment (see Figure 4 above).
Option 3. For amputated limbs and spleens or other large biopsies, submit the intact specimen chilled and fresh, and do not fix in formalin. The specimen should be wrapped in saline-soaked gauze or towels, placed in a sealed plastic bag, and refrigerated (NOT FROZEN) until transported. Coldpacks should accompany the sample. Specimens should be transmitted to the diagnostic laboratory as rapidly as possible, to limit autolysis.
Advantage: simple.
Disadvantage: increased autolysis, especially if chilling and shipping is not prompt. Results will be delayed by at least a day because tissue must be fixed after the laboratory receives the sample.
Please phone the Animal Health Laboratory @ 519-824-4120 ext. 54530 or email ahlinfo@uoguelph.ca [1] if you have questions regarding specific biopsy circumstances.