Emily Brouwer, Davor Ojkic
Animal Health Laboratory, University of Guelph, Guelph, ON.
AHL Newsletter 2024;28(1):19.
The bovine abortion PCR panel was made available in 2018 as a means to bundle testing for abortogenic pathogens in bovine specimens. This PCR detects bovine herpesvirus-1 (BoHV-1), Leptospira spp., and Neospora caninum, and can be performed on fetal tissues (kidney, liver spleen) or placenta. In cases where only formalin-fixed paraffin-embedded tissue is available, the PCR can also be performed on scrolls of tissue blocks. This test is routinely included in the diagnostic workup when fetuses are submitted to the lab or can be ordered when submitting specimens from field postmortems.
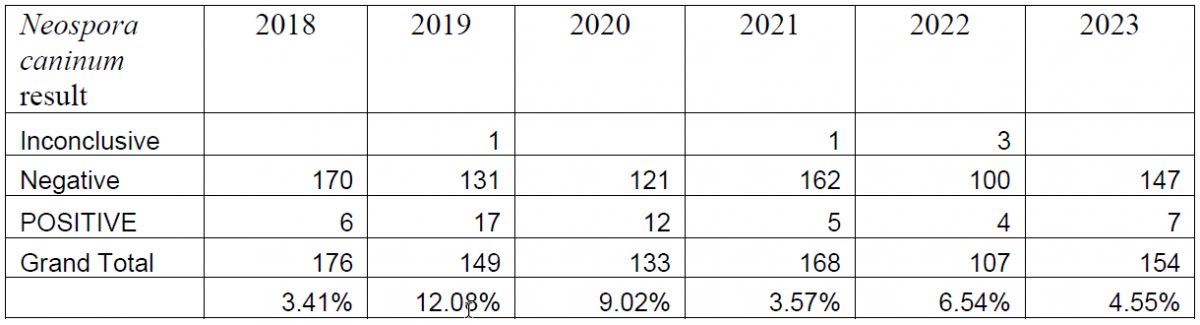
Between the introduction of the PCR in 2018 and the end of 2023, a total of 887 bovine abortion PCR panels have been performed. Overall, BoHV-1 has been detected in 5.02% of the submitted tests, Leptospira spp. has been detected in 1.72% of submissions, and Neospora caninum has been detected in 6.53% of submissions (Tables 1, 2, 3).
Table 1. Summary of bovine abortion panel PCR results for bovine herpesvirus-1 (2018-2023).
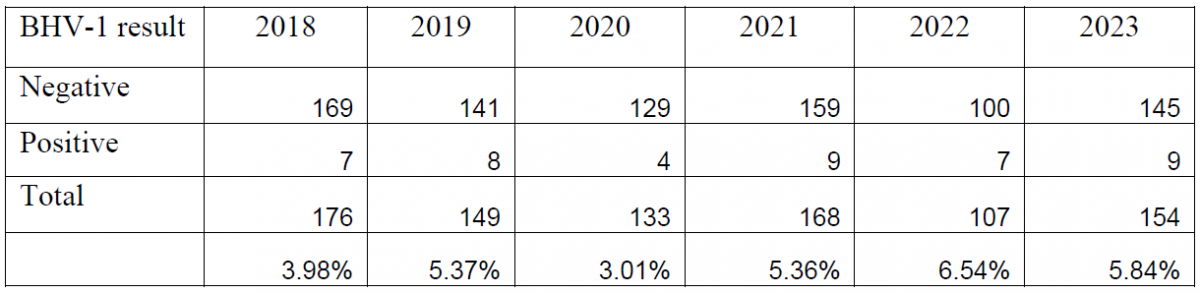
Table 2. Summary of bovine abortion panel PCR results for Leptospira spp. (2018-2023).
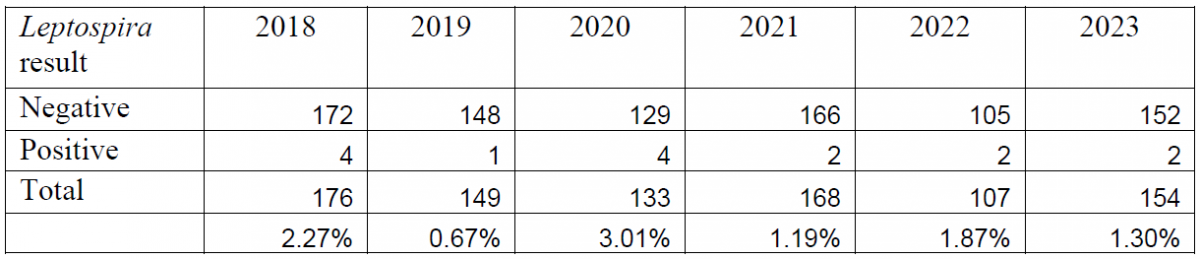
Table 3. Summary of bovine abortion panel PCR results for Neospora caninum (2018-2023).
When bovine abortion cases are submitted to the Animal Health Laboratory, the bovine abortion PCR panel is typically run in conjunction with other ancillary testing, including postmortem examination, histology, bacterial culture, other PCRs (BVDV, Ureaplasma), and Leptospira microagglutination testing. When sent in from external clients, this panel is often submitted as a stand-alone test, which can lead to some challenges in interpretation as detection of an agent does not necessarily constitute an etiologic diagnosis.
In cases of BoHV-1, it was noted that when histology was performed in conjunction with the PCR test, almost all positive cases had characteristic histologic lesions. In one case where BoHV-1 was detected with a relatively high cycle threshold, there were no characteristic herpesviral lesions on microscopic examination, and abortion was attributed to bacterial infection. In this case, the PCR was repeated on fetal liver and was negative.
Diagnosis of abortion due to leptospirosis proved to be more challenging. Although detected by PCR, many cases did not have characteristic histologic lesions, or had non-specific lesions and required follow-up testing. In most cases where Leptospira spp. was detected, AHL pathologists were able to perform additional testing (Leptospira microagglutination (MAT) testing and immunohistochemistry (IHC)) to further investigate the potential contribution of this pathogen to abortion. In cases where Leptospira infection was determined to be the cause of abortion, the diagnosis was usually confirmed with IHC or MAT. Of all the positive cases, only one test was submitted as a send-in, and no confirmatory testing was performed.
In cases where Neospora caninum was detected, most cases where histology was available had either characteristic or suggestive microscopic lesions. Rarely, histology was not performed due to the state of preservation of the fetus. Two send-in cases included tissues for histology as well; one of which had characteristic histologic lesions, and the other only included placenta with no fetal tissues. In rare cases where the PCR was inconclusive, pathologists were able to confirm the diagnosis either with routine histology, IHC, or Neospora caninum ELISA.
The bovine abortion PCR panel has become an integral part of the work up for bovine pregnancy loss. We encourage veterinarians investigating abortions in bovine herds to include formalin-fixed tissues for microscopic examination, as well as various fresh fetal tissues and maternal serum for additional testing (if indicated). The Animal Health Laboratory User’s Guide and Fee Schedule provides sampling advice, if needed. AHL