Durda Slavic
Part 3 – Selection of antimicrobials
Part 4 – Laboratory results interpretation
Part 1 – Test methods
When performing antimicrobial susceptibility testing (AST), all diagnostic labs in North America are expected to follow the standards and guidelines established by Clinical Laboratory Standard Institute (CLSI). The goal of the institute is to promote accurate and reproducible AST results, as well as appropriate reporting and interpretation by standardizing all aspects of AST. A subcommittee on veterinary antimicrobial susceptibility testing (VAST) establishes guidelines for veterinary laboratories including testing methods, bacterial density, media and drug types, the drug dilutions, incubation times, QC requirements, and - most importantly - interpretative criteria. Some of these recommendations are discussed below.
Test methods
There are two different methods frequently used by veterinary diagnostic laboratories to determine in vitro susceptibility of bacteria to antimicrobial agents: disk diffusion and microbroth dilution.
- The disk diffusion test, also known as Kirby Bauer (KB), is a test where a standardized bacterial suspension is plated on different plate types and disks impregnated with specific antimicrobials are placed on top of it. The drug concentrations on the disks are based on plasma steady-state drug concentrations in humans after IV dosing, although many have now been assessed in veterinary species (see Part 5). However, other than as indicated for bovine mastitis, the pharmacology may not reflect the relevant site of infection. After a specific incubation time, the inhibition of bacterial growth is measured and expressed in millimeters (Fig. 1).
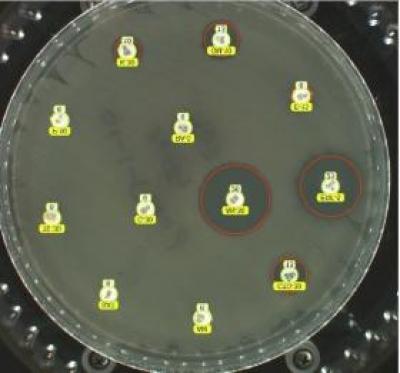
Fig. 1. Disk diffusion plate demonstrating disks impregnated with antibiotics placed on an agar plate with the bacterial species of interest. Note the red circles highlighting the zones of inhibited bacterial growth.
- Microbroth dilution, also known as minimal inhibitory concentration (MIC), is a test where a standardized bacterial suspension is dispensed on a 96 well commercially available plate with each well containing different dilutions of antimicrobial agents. After a specific incubation time, the first well with no growth (Fig. 2) in a series of wells with different antimicrobial concentrations of the same agent denotes the minimal inhibitory concentration and it is expressed in µg/ml. MIC data need to be interpreted with pharmacokinetic data from the literature or the antimicrobial product data to estimate the drug concentration that is achievable at the site of infection.

Fig. 2. Microbroth dilution plate demonstrating a series of wells containing “buttons” of bacteria growing in broth. The first well in each row containing no growth (button) indicates the minimal inhibitory concentration for that particular antibiotic. For example, red square in row F.
Part 2 – Bacterial organisms
- It is acceptable to proceed with empirical treatment for bacterial species that have a predictable susceptibility with low level or no known resistance to certain drugs.
- Penicillin is the recommended treatment choice for beta haemolytic streptococci including Streptococcus equi subsp. equi, S. zooepidemicus, and S. canis. It is also a preferred treatment option for Trueperella pyogenes.
- It is recommended to test a bacterial organism that contributes to an infectious process that requires treatment if its susceptibility cannot be reliably predicted based on bacterial ID.
- AST is most often indicated for bacterial species known to have the ability to develop resistance to commonly used antimicrobial agents (i.e., E. coli, Staphylococcus spp., Pseudomonas aeruginosa).
- Both AST methods have been developed for routine testing of fast-growing aerobic bacteria and are modified for testing of some fastidious bacteria such as Actinobacillus pleuropneumoniae, Mannheimia haemolytica and Histophilus somni.
- It is discouraged to perform AST on normal bacterial flora such as coagulase negative Staphylococcus spp. from skin samples.
- CLSI guidelines are not available for all bacterial species.
- Breakpoints are not established for certain veterinary pathogens because they may be difficult to grow under standard testing conditions (i.e., Glaesserella (Haemophilus) parasuis), they require a higher biosafety level than may be available in veterinary diagnostic labs (i.e., Brucella spp.), or if they are newly discovered.
Part 3 – Selection of antimicrobials
- Ideally, only one representative of the drug category which has similar efficacy as the other drugs in the same group needs to be tested. Table 1 indicates which drugs are typically tested on behalf of the entire group, and which drugs have to be tested individually.
- Only non-proprietary or generic drug names are used.
- Preferably, drugs should be approved for use in specific animal species and for specific disease processes.
| Drugs | Predicts susceptibility to |
| Ampicillin |
Amoxicillin Amoxicillin-clavulanate and amoxicillin-sulbactam (only if ampicillin is S * |
| Cephalexin | 1st generation cephalosporins |
| Cephalothin | 1st generation cephalosporins |
| Clindamycin | Lincomycin |
| Gentamicin | All aminoglycosides, except streptomycin |
| Tetracycline | Doxycycline, minocycline, and oxytetracycline (only if tetracycline is S *) |
| Trimethoprim- sulfamethoxazole | Trimethoprim-sulfadiazine |
| Sulfisoxazole | Sulfonamides |
| Erythromycin | Azithromycin and clarithromycin (only if erythromycin is S*) |
Reprinted with permission from Clinical and Laboratory Standards Institute from: CLSI. Understanding Susceptibility Test Data as a Component of Antimicrobial Stewardship in Veterinary Settings. 1st ed. CLSI report VET09. Wayne, PA: Clinical and Laboratory Standards Institute; 2019. *S=susceptible. If results for these drugs are R (resistant), then susceptibility to the other specified drugs cannot be predicted and they have to be tested individually.
Part 4 – Laboratory results interpretation
When it comes to interpretation of AST results, CLSI provides a breakpoint for different drugs which in turn is translated into an interpretative category (i.e., S, I, and R). As per CLSI: “breakpoint is the minimal inhibitory concentration or zone diameter value used to categorize an organism as susceptible,
intermediate or resistant”. Whenever available, the VAST subcommittee recommends use of veterinary specific clinical breakpoints because they are determined based on microbiological characteristics, pharmacokinetic and pharmacodynamics parameters and/or clinical outcome (Table 2). Veterinary specific clinical breakpoints are not available, however, for every drug/bacterial species/disease combination. At present, there are veterinary specific guidelines available for some veterinary pathogens associated with urinary tract infections, primarily in dogs and cats, respiratory infections in cattle and swine, mastitis in cattle, metritis, abscesses, wounds, skin and soft tissue infections. In some cases, human clinical breakpoints are still used or results are extrapolated from similar veterinary drug/bacterial species/disease combinations (i.e., M. haemolytica respiratory breakpoints are used to predict susceptibility of Biberstainia trehalosi in bovine respiratory samples).
Here is what it means to get S, I, or R results as per CLSI:
“Susceptible (S) – a category defined by a breakpoint that implies that isolates with an MIC at or below, or a zone diameter at or above, the susceptible breakpoint are inhibited by the usually achievable concentrations of antimicrobial agent when the dosage recommended to treat the site of infection is used, resulting in likely clinical efficiency.”
“Intermediate (I) – a category defined by a breakpoint that includes isolates with MICs or zone diameters within the intermediate range that approach usually attainable blood and tissue levels and for which response rates may be lower than for susceptible isolates. The intermediate category implies clinical efficacy in body sites where the drugs are physiologically concentrated or when a higher than normal dosage of drug can be used. This category also serves as a buffer zone to prevent the inherent variability of antimicrobial susceptibility testing methods from leading to erroneous categorization.”
“Resistant (R) – a category defined by a breakpoint that implies that isolates with an MIC at or above, or a zone diameter at or below, the resistant breakpoint are not inhibited by the usually achievable concentrations of the agent within normal dosage schedules and/or that specific microbial resistance mechanisms are likely, and clinical efficacy of the agent against the isolate has not been reliably shown in isolates with similar phenotypes.”
Table 2. List of antimicrobial agents that have CLSI-approved veterinary specific breakpoints.
| Antimicrobial agent | Canine | Feline | Equine | Bovine | Bovine mastitis | Swine |
| Amikacin | X | X | ||||
| Gentamicin | X | X | ||||
| Spectinomycin | X | |||||
| Amoxicillin-clavulanate | X | X | ||||
| Ampicillin | X | X | X | X | X | |
| Cefazolin | X | X | ||||
| Cefovecin | X | X | ||||
| Ceftiofur | X | X | X | X | ||
| Cephalexin | X | |||||
| Cephalothin | X | |||||
| Penicillin G | X | X | X | |||
| Penicillin-novobiocin | X | |||||
| Danofloxacin | X | |||||
| Difloxacin | X | |||||
| Enrofloxacin | X | X | X | X | X | |
| Marbofloxacin | X | X | ||||
| Orbifloxacin | X | X | ||||
| Pradofloxacin | X | X | ||||
| Clindamycin | X | X | ||||
| Pirlimycin | X | |||||
| Gamithromycin | X | |||||
| Tildipirosin | X | X | ||||
| Tilmicosin | X | X | ||||
| Tulathromycin | X | X | ||||
| Doxycycline | X | X | ||||
| Minocycline | X | X | ||||
| Tetracycline | X | X | X | |||
| Florfenicol | X | X | ||||
| Tiamulin | X |
Reprinted with permission from Clinical and Laboratory Standards Institute from: CLSI. Understanding Susceptibility Test Data as a Component of Antimicrobial Stewardship in Veterinary Settings. 1st ed. CLSI report VET09. Wayne, PA: Clinical and Laboratory Standards Institute; 2019.
Part 5 – Results reporting
As per CLSI: “Veterinarians should understand that the presence of AST results for a drug on a laboratory report does not provide permission or the necessary justification for use of that drug. The presence of the drug on the panel does not indicate that it is legal or appropriate to use.” It is the responsibility of a veterinarian to use the drugs as per their label recommendations, as laboratories do not provide separate panels for different commodity groups (i.e., beef vs dairy), and sometimes one antimicrobial panel is shared with different animal species (e.g. bovine/porcine panel).
If the drug is excluded from the report, it is likely that:
-
- The bacterial organism is intrinsically resistant to it and there is no need to perform AST (Table 3);
- there are no CLSI guidelines available for that particular organism/drug combination; or
- the drug is not approved for use in that food animal species.
Table 3. Examples of intrinsic resistance in Enterobacterales and Enterococcus spp.
| Organism | Antimicrobial agent |
| Enterobacterales | Clindamycin, fusidic acid, glycopeptides, macrolides, rifampin |
| Enterococcus spp. | Aminoglycosides, cephalosporins, clindamycin, trimethoprim, trimethoprim-sulfamethoxazole |
Reprinted with permission from Clinical and Laboratory Standards Institute from: CLSI. Understanding Susceptibility Test Data as a Component of Antimicrobial Stewardship in Veterinary Settings. 1st ed. CLSI report VET09. Wayne, PA: Clinical and Laboratory Standards Institute; 2019.
References:
- CLSI. Performance standards for antimicrobial disk and dilution susceptibility tests for bacteria isolated from animals. 5th ed. CLSI supplement VET01S. Clinical and Laboratory Standard Institute; 2020.
- CLSI. Understanding susceptibility test data as a component of antimicrobial stewardship in veterinary settings. 1st ed. CLSI report VET09. Clinical and Laboratory Standard Institute; 2019.