AHL LabNote 44-Testing algorithm for suspected lymphoid neoplasms (clonality, PARR)
Dorothee Bienzle, Rebecca Egan, Department of Pathobiology, University of Guelph
Below is a proposed step-wise algorithm for challenging or suspected lymphoid neoplasms available in concert with the AHL. The approach capitalizes on complementarity of methods.
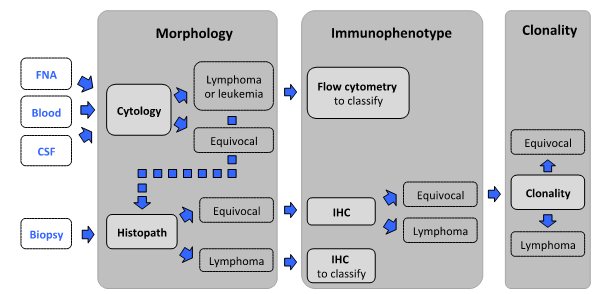
Morphology - The first step is morphologic assessment of specimens, by cytology or histopathology.
Cytology: For initial assessment of enlarged lymph nodes or effusions, cytology is the test of choice because it is minimally invasive, cost-effective, and can identify lymphoid neoplasia in most cases in dogs, and about half of cats and horses. Tissue architecture cannot be assessed by cytology.
Biopsy: Histopathology is the appropriate test when cytologic findings are equivocal, when concurrent assessment of tissue architecture is necessary (gastrointestinal and skin lesions, spleen), and to classify lymphoid neoplasia.
Immunophenotyping is indicated when cytology and/or histopathology are equivocal or to classify lymphoid neoplasia.
Flow cytometry (FC): Cells in fresh tumor samples are analyzed for light scatter and expression of a range of markers. Cells are not assessed microscopically in FC; therefore the test should only be used in conjunction with cytology.
Immunohistochemistry (IHC): In formalin-fixed paraffin-embedded tissues, IHC is used to identify the 2 major lymphocyte subsets, B and T lymphocytes. This aids in the assessment of complex lesions and it is required for morphologic classification of lymphoid neoplasms and for the interpretation of clonality testing results.
Clonality testing can help distinguish reactive from neoplastic lymphoid proliferations if other methods are equivocal.
Clonality results must be interpreted in conjunction with clinical, morphologic, and immunophenotypic data, and should hence be done as the last step in the diagnostic algorithm. Clonality testing is not suitable for immunophenotyping. Clonality testing from cytology samples is feasible if a biopsy cannot be obtained readily (e.g., CSF, effusions without primary mass, etc.). Ideally, additional material should be obtained for immunophenotyping (flow cytometry, immunochemistry). The latter information is required for the interpretation of clonality results. Samples without immunophenotyping will not be accepted, with the exception of samples where the immunophenotype is apparent base on cytomorphology (i.e. marginal cell proliferations or intestinal small cell infiltrates).
Submission of samples for flow cytometry (dog, cat, horse)
Suitable samples
- Whole blood or bone marrow in EDTA (purple top) tube
- CSF in red top tube
- Lymph node, spleen, liver, etc. aspirate with 2-4 mL of saline in EDTA tube
Technique
- Blood and bone marrow: Place 1-2 mL of sample in EDTA tube. Prepare one featheredge smear and air dry.
- CSF: Collect 0.5 to 2 mL of sample with cell count of at least 5 cells/uL. Add 1-2 drops of serum (from same dog or other dog)
- Fine needle aspirate: Collect as usual with a 22 or 23 g needle. Four to 5 passages through tissue are usually needed to get enough cells. Prepare one featheredge smear and air dry. Place remainder of sample in EDTA tube containing 2-4 mL of saline plus 2-4 drops of serum. Rinse needle with saline. Fluid should appear cloudy indicating presence of cells.
Shipping
- Label samples and slides.
- Samples are suitable for analysis for maximally 3 days stored at refrigeration.
- Ship on ice pack Monday to Thursday.
- Do not allow samples to freeze.
- Enclose ancillary information – results of CBC, CSF analysis, etc.
Animal Health Laboratory
Attention: Flow Cytometry Laboratory
University of Guelph
Building 89, NW Corner Gordon/McGilvray
Guelph, Ontario N1G 2W1
For further information: dbienzle@uoguelph.ca, 519-824-4120 x54351
Submission of samples for clonality testing (dog, cat)
Clonality testing is currently unavailable. During this time, please direct any clonality-related questions or inquires to Dr. Rebecca Egan (eganr@uoguelph.ca OR ext. 56712)
All submitted samples must have assessment from a pathologist, and all required materials and information must be submitted with all submissions.
Required information
- Clinical history
- H&E or cytology slides (digital access is acceptable)
- IHC slides (digital access is acceptable), flow cytometry data, immunocytochemistry (ICC) slides
Suitable samples for clonality testing
- Tissues: fresh/frozen
- Scrolls (from FFPE tissue blocks): 2 sets of 3 x 5 μm in separate tubes OR unstained sections (six 5 μm sections, not “baked” & on uncharged slides)
- Cytology slides: stained or unstained (note these will not be returned)
- Fluids: see submission information for flow cytometry samples
Shipping
- Submit to the AHL using the Companion Animal Submission Form for Clonality Testing
- If IHC has not been done previously, please submit the FFPE block or 6 unstained sections on positively charged slides. An additional fee will apply for IHC.
- All submitted materials will be reviewed by a pathologist, and findings will be integrated for a final diagnosis.
For further information: eganr@uoguelph.ca, 519-824-4120 x56712




