RUMINANTS
Outbreak of parasitic pneumonia in a herd of beef cattle
Jan Shapiro, Andrew Peregrine, Jeff Caswell
In late June 2017, 2 mature beef cows from a herd of 61 cows and calves in eastern Ontario were submitted for postmortem to the AHL-Kemptville. Five cows had died in 11 days, and many others had a raspy cough. The calves were not affected. The herd was not vaccinated or dewormed. The cattle were kept on pasture, with access to a run-in area in a barn.
Cow A had been ill for 2 days, but was not treated and died. Cow B was the index case in the herd, and had been sick for 11 days. She was not treated and seemed to be improving gradually, but died unexpectedly. At postmortem, cow A was in very thin body condition, and cow B was emaciated. Significant gross lesions were confined to the lungs. The cranial and middle lung lobes of both cows and the cranioventral 1/2 of the right and left caudal lobes of cow B were mottled red and dark-red, and had a rubbery-to-firm texture. It was estimated that 55% of the lung mass of cow B was affected. Interlobular septa were mildly distended with edema or emphysema. No exudate could be expressed from airways. Bronchial lymph nodes were markedly diffusely hyperplastic.
The postmortem diagnosis was acute interstitial pneumonia. PCR testing for bovine respiratory syncytial virus, parainfluenza 3 virus, and bovine herpesvirus 1 was negative.
Histopathology of the lungs of both cows was dramatic. The lumen of alveoli and bronchioles was filled with eosinophils, non-degenerated neutrophils, macrophages, and multinucleate giant cells. Alveolar hyaline membranes and fibrin balls were prominent, and there was multifocal acute alveolar hemorrhage. Alveolar type 2 hyperplasia was marked and there were scattered syncytial cells. Interalveolar septa were expanded by lymphocytes and eosinophils; septal fibrosis was mild to severe. Eosinophils were in bronchiolar lamina propria, and transmigrating bronchiolar epithelium. In one cow, occasional bronchioles had focal attenuation or hyperplasia of lining epithelium, some were ruptured, and some had peribronchial fibrosis. Edema and a diffuse infiltrate of eosinophils admixed with smaller numbers of neutrophils and macrophages expanded interlobular septa and pleural lymphatics. There was multifocal parenchymal caseous necrosis and eosinophilic granulomas, which were occasionally mineralized. Necrotic and inflammatory foci were often centered on intact or degenerating nematode parasites. Parasites had an eosinophilic cuticle, with 2 spines (alae) and a large intestinal tract (Fig. 1), and were interpreted as ascarid larvae. The histologic diagnosis was severe parasitic bronchointerstitial pneumonia.
Dictyocaulus viviparus, the pathogenic bovine lungworm, is a trichostrongyle. However, it is unusual to see this parasite cause disease in Ontario in June and in cattle that have been on pasture for more than one grazing season. Furthermore, the morphology of nematodes in the lungs of these cattle was more consistent with ascarids. Additional history subsequently provided by the owner was that in one corner of the run-in for the cattle, there was a manure pile that had been used for the past 2 years to store manure from the pen of a small group of pigs that were kept in an adjacent area of the barn. The cattle had access to this manure pile.
The history of access to pig manure, and the histologic lesions are consistent with a presumptive diagnosis of aberrant Ascaris suum migration, but unambiguous identification of the parasites would require gene sequencing of the parasite.
After receiving the postmortem report, the owner dewormed the cattle, restricted access to the pig manure pile, and reported that there have been no new cases of respiratory disease. AHL
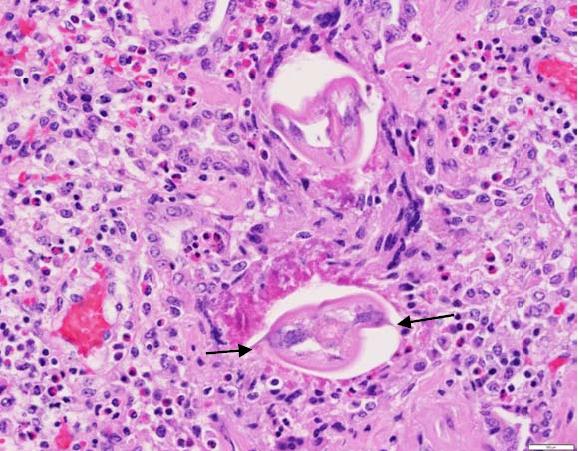
Figure 1. Lung section showing eosinophilic inflammation and cross section of parasites. Note lateral alae (arrows).




